Différences entre les versions de « VBTutorial3 »
| Ligne 44 : | Ligne 44 : | ||
# Matrix element between determinants differing by spin inversion of two spin-orbitals : | # Matrix element between determinants differing by spin inversion of two spin-orbitals : | ||
| − | == Exercice 2 | + | == Exercice 2 : computation of H—H + H. -> H. + H—H hydrogen exchange state correlation diagram == |
=== Subject === | === Subject === | ||
Here is a image example [[File:Allyl_cation_MO.png|thumb|right| 100px|alt=Example alt text |Image example: Allyl Cation MO's]] | Here is a image example [[File:Allyl_cation_MO.png|thumb|right| 100px|alt=Example alt text |Image example: Allyl Cation MO's]] | ||
| Ligne 54 : | Ligne 54 : | ||
[[VBFile 4-2 | title]] | [[VBFile 4-2 | title]] | ||
| − | == Exercice 3 | + | == Exercice 3 : Computation of state correlation Diagrams in 3 centers / 4 electrons systems == |
Version du 25 mai 2012 à 08:40
How to modify this page :
- first : log in (top right of this page) ;
- click on [edit] (far right) to edit a section of the page ;
- write your text directly in the wiki page, and click on the "Save page" button (bottom left) to save your modifications
See also this page for an introduction to the basics of the wiki syntax
To the Tutors
Sason remarks and prospective 2 hours talk +
Philippe's remark on the initially proposed tutorial. are included in bold.
Qualitative
- State correlation Diagrams in 3 centers systems : H3C. - H. - .CH3 (or 4 electrons .... H transfer barrier appearing in F H F(-) when the F ... F distance is increased (which avoids geometry problems).
Yes, but we could start with H3
- Benzen pi system dissymetrization (2 geometries (D6h and D3h); on each geometry 2 spin alternant determinants cf p154 on The Book) - Pbm : check how differentiate sigma vs + (sigma + pi) energies.
Objection : Using two spin-alternant determinants refers to Heisenberg’s spin hamiltonian theory, that we don’t teach. Differentiating sigma vs sigma + pi energies is a bit off-topic.
Exercices
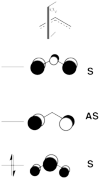
Exercice 1 (paper exercice) : Conical intersection in H3. radical
Consider three hydrogen atoms Ha, Hb, Hc, with respective atomic orbitals a, b and c, and the two VB structures R = and P = . The Ha-Hb and Hb-Hc distances are equal.
- By using the thumb rules recalled below, where squared overlap terms are neglected, derive the expression of the energies of R and P, and of the reduced Hamiltonian matrix element between R and P for the 3-orbital/3-electrons reacting system [Ha--Hb--Hc]•.
- From the sign of this latter integral when θ > 60°, derive the expressions of the ground state Ψ≠ and of the first excited state Ψ* of the H3• system. One may drop the normalization constants for simplicity. What bonding scheme does the excited state represent ?
- Show that the reduced Hamiltonian matrix element is largest in the collinear transition state geometry, and drops to zero in the equilateral triangular structure.
- Show that R and P VB structures are degenerate in the equilateral triangular structure, and that Ψ≠ and Ψ* are also degenerate in this geometry.
- We now extend the above conclusions to the allyl radical. What are the bonding schemes corresponding to the ground state and first excited state ? What geometrical distortion would make these two states degenerate ? What would be the end product of a photochemical excitation of allyl radical to its first excited state ?
Appendix : Thumb rules for the calculations of effective Hamiltonian matrix elements between determinants.
- Energy of a determinant D : ... (if orbitals i and j have parallel spins)
- Matrix element between determinants differing by spin inversion of two spin-orbitals :
Exercice 2 : computation of H—H + H. -> H. + H—H hydrogen exchange state correlation diagram
Subject
Here is a image example