Différences entre les versions de « Orals CTTC2019 »
| (9 versions intermédiaires par 2 utilisateurs non affichées) | |||
| Ligne 2 : | Ligne 2 : | ||
| − | <big>PLEASE UPLOAD YOUR ABSTRACT BEFORE 28TH FEBRUARY</big> | + | <big> |
| + | PLEASE UPLOAD YOUR ABSTRACT BEFORE 28TH FEBRUARY | ||
| + | |||
| + | DECISIONS WILL BE COMMUNICATED DURING MARCH TO FACILITATE REGISTRATION | ||
| + | </big> | ||
| + | |||
| − | |||
| Ligne 25 : | Ligne 29 : | ||
* [[How to insert a picture| >>> How to insert a picture]] in your abstract | * [[How to insert a picture| >>> How to insert a picture]] in your abstract | ||
| + | =Orals Proposals= | ||
| + | |||
| + | ==Raúl Quintero Monsebaiz== | ||
| + | ''CINVESTAV'' | ||
| + | |||
| + | '''<big>Spin polarized Natural Orbital functional theory</big>''' | ||
| + | |||
| + | Within Natural Orbital Functional Theory a spin-uncompensated formulation for an independent pair approach PNOF5[1][2] and an interacting pair model PNOF7[3][4] was proposed, each one of these functionals fulfills the basic properties of the first- and second-order reduced density matrices. The spin symmetry also is accomplished taking into account the conservation of the total spin. A benchmarking was made using the one dimensional Hubbard Model with periodic boundary conditions[5], the spin-polarized functionals show the correct description of strong-correlation effects. | ||
| + | |||
| + | |||
| + | [1] M. Piris, X. Lopez, F. Ruipérez, J. M. Matxain, and J. M. Ugalde, J. Chem. Phys. 134, 164102 (2011). | ||
| + | |||
| + | [2] M. Piris, J. M. Matxain, and X. Lopez, J. Chem. Phys. 139, 234109 (2013). | ||
| + | |||
| + | [3] M. Piris, Phys. Rev. Lett. 119, 063002 (2017). | ||
| + | |||
| + | [4] I. Mitxelena, M. Rodríguez-Mayorga, and M. Piris, Eur. Phys. J. B91, 109 (2018). | ||
| + | |||
| + | [5] R Quintero, I. Mixtelena,M. Rodriguez,A. Vela, M. Piris, J. Phys: Condens Matter, (2019) | ||
| + | |||
| + | |||
| + | [https://wiki.lct.jussieu.fr/workshop/index.php/Abstracts_of_the_CTTC_2016 ↑ top of this page] | ||
| + | |||
| + | |||
| + | ==Fernando Mendizabal== | ||
| + | ''Universidad de Chile'' | ||
| + | |||
| + | '''<big>Electronic and Optical Properties of the [Au(ditioacetate)]4 clusters</big>''' | ||
| + | |||
| + | Noncovalent interactions between molecules can lead to the emergence of large structures. It is possible to go from the molecular to the supramolecular system's chemistry, which aims to develop chemical systems highly complex through intra- and intermolecular forces. In the last time, within this area, inorganic supramolecular systems chemistry has been developed. Those systems have a structural orientation which is defined by certain forces that predominate in the associations among molecules. It is possible to recognize these forces as hydrogen bonding, stacking, halogen bonding, electrostatic, hydrophobic, charge transfer, metal coordination, and metallophilic interactions. The presence of these forces in supramolecular system yields certain properties such as light absorption and luminescence. Quantum theoretical modeling plays an important role in the designing of the supramolecular system. The goal is to apply supramolecular principles in order to understand the associated forces in many inorganic molecules that include heavy metals for instance gold, platinum, and mercury. Within this broad strand of systems, gold(I) chalcogenides represent an important class of luminescent gold(I) clusters. The gold thiolate cluster [Au(dta)]4 (dta = dithioacetate), in which the four gold centers are arranged to form a rhomb. We have proposed a theoretical study based on the quantum mechanics at ab initio post Hartree-Fock (MP2 and CC2) and Density Functional Theory (DFT) with dispersion | ||
| + | |||
| + | |||
| + | [https://wiki.lct.jussieu.fr/workshop/index.php/Abstracts_of_the_CTTC_2016 ↑ top of this page] | ||
| + | |||
| + | =Posters proposals= | ||
| + | |||
| + | ==Joakim Halldin Stenlid== | ||
| + | ''Department of Physics, Stockholm University, Sweden'' | ||
| + | |||
| + | |||
| + | '''<big>Introducing Regium Bonds</big>''' | ||
| + | |||
| + | When interacting with electron-donors, neutral compounds of copper, silver and gold form bonds that are similar to hydrogen and halogen bonds [1]. This type of bonding is referred to as regium bonding and it have been used to rationalize e.g. noble metal catalysis [2-3]. Compounds donating regium, halogen and hydrogen bonds have in common local regions deprived in electron density, known as σ-holes. The chemistry of such regions can be characterized by local maximum in the electrostatic potential evaluated on contour surfaces of constant electron density (VS,max); the position of the VS,max identifies sites susceptible to interactions with nucleophiles, e.g. H2O, H2S, NH3 and CO, while the magnitude of VS,max scales with the strength of the interaction. By this approach, information on the reaction and interaction tendencies of a compound can be swiftly accessed by standard DFT calculations. Thus poster will introduce regium bonds, exemplify its relevance in materials and nanoparticle science. | ||
| − | + | <center>[[File:RBond.png|500px]]</center> | |
| − | + | Figure. Left, the electrostatic potential plotted on an 0.001 au isodensity surface of a icosahedral Au nanoparticle. Red > yellow > green sites mark VS,max (σ-holes) susceptible to interactions with nucleophiles (i.e. to the formation of regium bonds). Right, comparison of the regium, halogen and hydrogen bonds with Nu being an nucleophile. e.g. H2O or NH3. | |
| − | |||
| − | + | [1] JH Stenlid, AJ Johansson and T Brinck, σ-Holes and σ-Lumps Direct the Lewis Basic and Acidic Interactions of Noble Metal Nanoparticles: Introducing Regium Bonds, Physical Chemistry Chemical Physics, (2018), 20, 2676–2692 | |
| + | [2] JH Stenlid and T Brinck, Extending the σ-Hole Concept to Metals: An Electrostatic Interpretation of the Effects of Nanostructure in Gold and Platinum Catalysis, Journal American Chemical Society, (2017), 139, 11012-11015 | ||
| − | + | [3] T Brinck, JH Stenlid, The Molecular Surface Property Approach: A Guide to Chemical Interactions in Chemistry, Medicine, and Material Science, Advanced Theory and Simulations, (2019), 2, 1800149 | |
| − | |||
[https://wiki.lct.jussieu.fr/workshop/index.php/Abstracts_of_the_CTTC_2016 ↑ top of this page] | [https://wiki.lct.jussieu.fr/workshop/index.php/Abstracts_of_the_CTTC_2016 ↑ top of this page] | ||
Dernière version du 29 avril 2019 à 20:04
<<< CTTC 2019 workshop main page
PLEASE UPLOAD YOUR ABSTRACT BEFORE 28TH FEBRUARY
DECISIONS WILL BE COMMUNICATED DURING MARCH TO FACILITATE REGISTRATION
HOW TO UPLOAD YOUR ABSTRACT
In order to upload your abstract, you will need a valid login account.
Please contact us email and we will email you your login details.
Please be aware that the whole system has been reinitialized, so old accounts are not valid anymore.
CONTRIBUTORS: please add below, in your own section, your title talk and abstract :
- first : log in; (contact the organization for log in details or use the ones we provided in 2014 if you attended Vietnam)
- click on your name in the "Contents" box below, this will lead you to your own section;
- your section starts with your name as the title line, click on [edit] (far right).
- >>> How to insert a picture in your abstract
Orals Proposals
Raúl Quintero Monsebaiz
CINVESTAV
Spin polarized Natural Orbital functional theory
Within Natural Orbital Functional Theory a spin-uncompensated formulation for an independent pair approach PNOF5[1][2] and an interacting pair model PNOF7[3][4] was proposed, each one of these functionals fulfills the basic properties of the first- and second-order reduced density matrices. The spin symmetry also is accomplished taking into account the conservation of the total spin. A benchmarking was made using the one dimensional Hubbard Model with periodic boundary conditions[5], the spin-polarized functionals show the correct description of strong-correlation effects.
[1] M. Piris, X. Lopez, F. Ruipérez, J. M. Matxain, and J. M. Ugalde, J. Chem. Phys. 134, 164102 (2011).
[2] M. Piris, J. M. Matxain, and X. Lopez, J. Chem. Phys. 139, 234109 (2013).
[3] M. Piris, Phys. Rev. Lett. 119, 063002 (2017).
[4] I. Mitxelena, M. Rodríguez-Mayorga, and M. Piris, Eur. Phys. J. B91, 109 (2018).
[5] R Quintero, I. Mixtelena,M. Rodriguez,A. Vela, M. Piris, J. Phys: Condens Matter, (2019)
Fernando Mendizabal
Universidad de Chile
Electronic and Optical Properties of the [Au(ditioacetate)]4 clusters
Noncovalent interactions between molecules can lead to the emergence of large structures. It is possible to go from the molecular to the supramolecular system's chemistry, which aims to develop chemical systems highly complex through intra- and intermolecular forces. In the last time, within this area, inorganic supramolecular systems chemistry has been developed. Those systems have a structural orientation which is defined by certain forces that predominate in the associations among molecules. It is possible to recognize these forces as hydrogen bonding, stacking, halogen bonding, electrostatic, hydrophobic, charge transfer, metal coordination, and metallophilic interactions. The presence of these forces in supramolecular system yields certain properties such as light absorption and luminescence. Quantum theoretical modeling plays an important role in the designing of the supramolecular system. The goal is to apply supramolecular principles in order to understand the associated forces in many inorganic molecules that include heavy metals for instance gold, platinum, and mercury. Within this broad strand of systems, gold(I) chalcogenides represent an important class of luminescent gold(I) clusters. The gold thiolate cluster [Au(dta)]4 (dta = dithioacetate), in which the four gold centers are arranged to form a rhomb. We have proposed a theoretical study based on the quantum mechanics at ab initio post Hartree-Fock (MP2 and CC2) and Density Functional Theory (DFT) with dispersion
Posters proposals
Joakim Halldin Stenlid
Department of Physics, Stockholm University, Sweden
Introducing Regium Bonds
When interacting with electron-donors, neutral compounds of copper, silver and gold form bonds that are similar to hydrogen and halogen bonds [1]. This type of bonding is referred to as regium bonding and it have been used to rationalize e.g. noble metal catalysis [2-3]. Compounds donating regium, halogen and hydrogen bonds have in common local regions deprived in electron density, known as σ-holes. The chemistry of such regions can be characterized by local maximum in the electrostatic potential evaluated on contour surfaces of constant electron density (VS,max); the position of the VS,max identifies sites susceptible to interactions with nucleophiles, e.g. H2O, H2S, NH3 and CO, while the magnitude of VS,max scales with the strength of the interaction. By this approach, information on the reaction and interaction tendencies of a compound can be swiftly accessed by standard DFT calculations. Thus poster will introduce regium bonds, exemplify its relevance in materials and nanoparticle science.
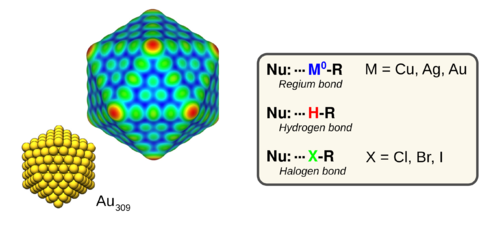
Figure. Left, the electrostatic potential plotted on an 0.001 au isodensity surface of a icosahedral Au nanoparticle. Red > yellow > green sites mark VS,max (σ-holes) susceptible to interactions with nucleophiles (i.e. to the formation of regium bonds). Right, comparison of the regium, halogen and hydrogen bonds with Nu being an nucleophile. e.g. H2O or NH3.
[1] JH Stenlid, AJ Johansson and T Brinck, σ-Holes and σ-Lumps Direct the Lewis Basic and Acidic Interactions of Noble Metal Nanoparticles: Introducing Regium Bonds, Physical Chemistry Chemical Physics, (2018), 20, 2676–2692
[2] JH Stenlid and T Brinck, Extending the σ-Hole Concept to Metals: An Electrostatic Interpretation of the Effects of Nanostructure in Gold and Platinum Catalysis, Journal American Chemical Society, (2017), 139, 11012-11015
[3] T Brinck, JH Stenlid, The Molecular Surface Property Approach: A Guide to Chemical Interactions in Chemistry, Medicine, and Material Science, Advanced Theory and Simulations, (2019), 2, 1800149