Différences entre les versions de « ELF abstracts page »
| Ligne 35 : | Ligne 35 : | ||
Abstract... | Abstract... | ||
| − | <center>[[File:PCH1.jpg| | + | <center>[[File:PCH1.jpg|250px]]</center> |
| − | <center>[[File:PCH2.jpg| | + | <center>[[File:PCH2.jpg|250px]]</center> |
'''References''' | '''References''' | ||
Version du 21 mai 2010 à 14:53
SPEAKERS : please add below, in your own section, your title talk and abstract :
- first : log in;
- click on your name in the "Contents" box below, this will lead you to your own section;
- your section starts with your name as the title line, click on [edit] (far right).
The order of abstract follow the program of the workshop.
E. Alikhani
Address
Title of the talk
Abstract...
References
[1]
[2]
P. Hiberty
Address
Title of the talk
Abstract...
References
[1]
[2]
C. Lepetit
Address
Title of the talk
Abstract...
References
[1]
[2]
M-M. Rohmer
Address
Title of the talk
Abstract...
References
[1]
[2]
B. De Courcy
Address
Title of the talk
Abstract...
References
[1]
[2]
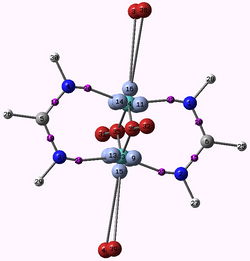
H. Jamet
Laboratoire Chimie Théorique, DCM, 301 Rue de la Chimie,Domaine Universitaire, 38041 Grenoble Cedex 9, email: hjamet@ujf-grenoble.fr
ELF and MESP : two efficient methods to validate a QM/MM partition for the modelization of Phosphine ligands
A fundamental question that arises after the decision of using QM/MM methods for a certain problem, is how to partition the system, which atoms are going to be included in the QM region and which atoms are going to be included in the MM region? Chemical knowledge is most of the time the guiding line for this choice, but tests should be run in case of uncertainty. In this study we discuss the modelization of phosphine ligands commonly used in the assymetric Pauson-Khand reaction[1] by means of QM/MM methods. X-ray structures of the ligands bound to the tungsten penta(tetra) carbonyl were used as starting point for all calculations. For the evaluation of the position of the QM/MM border, different approaches were used. Firstly, we compare the results of geometry optimizations obtained from ONIOM method using different QM-MM borders with those obtained from full quantum mechanical calculations. Secondly, we evaluate the molecular electrostatic potential (MESP) and the ELF function for the ligand alone, without optimizing it. More precisely we calculate the minimum value of the MESP around the lone pair region of the phosphorus atom as Suresh et al. done[2] and evaluate the population of the valence phosphorus basin V(P). We show that these two later approaches which have the advantage of being fast and computationally inexpensive reproduce the same trend than results obtained from geometry optimizations of the whole complex.
References
[1] K. Hiroi, T. Watanabe, R. Kawagishi and I. Abe. Tetrahedron: Asymmetry, 2000, 11:1197.
[2] J.Mathew, T. Thomas and C.H.Suresh, Inorg. Chem. 2007, 46(25):10800.
G. A. Cisneros
Address
Title of the talk
Abstract...
References
[1]
[2]
X. Assfeld
Address
Title of the talk
Abstract...
References
[1]
[2]
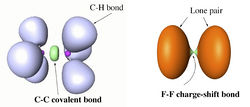
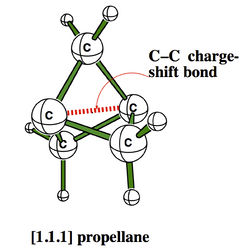
H. S. Rzepa
Department of Chemistry, Imperial College London, email: rzepa@imperial.ac.uk
ELF and the Nature of Triple bonds
A recent report of the species HOS≡CH by Schreiner, Mloston and co-workers1 speculated upon the nature of the SC bond; did it have triple significant bond character? Of the various techniques these authors used for the analysis, neither QTAIM nor ELF was employed. I initially filled this gap by a post on my blog2, and followed this by a full article submitted for publication more conventionally3. The results of this investigation will be presented at the workshop, including a rather surprising conclusion regarding the nature of triple bonds themselves in relation to the degree of charge-shift character they display4. The hypothesis so generated was then extended to investigating the nature of metal-metal multiple bonds, particularly Cr-Cr whose compounds are purported to exhibit homonuclear quadruple or quintuple bonds5. These systems also appear to sustain remarkably high charge-shift character and most intriguing ELF behaviour. The issue for discussion in the workshop is therefore whether or not ELF (combined with QTAIM) is indeed revealing something new about the nature of the triple and higher order homonuclear (metal) bond.
The talk will appear @www.ch.ic.ac.uk/rzepa/talks/elf10 and will feature rotatable ELF isosurfaces and models.
References
[1] P. R. Schreiner, H. P. Reisenauer, J. Romanski, and G. Mloston, Angew. Chemie. 2009, 48, 8133-8136. DOI: 10.1002/anie.200903969
[2] H. S. Rzepa, Blog and follow up
[3] H. S. Rzepa, submitted for publication, 2010.
[4] S. Shaik, D. Danovich, W. Wu and P. C. Hiberty, Nature Chem., 2009, 1, 443-449. DOI: 10.1038/nchem.327
[5] For initial speculations, see blog.
M. Yanez
Departamento de Química, C-9. Universidad Autónoma de Madrid. Cantoblanco, 28049-Madrid. Spain
ELF as a useful tool to understand chemical bonding in challenging cases
Quite unexpectedly, substituent effects on the structure of iminoboranes are opposite to those observed for the acetylene derivatives in spite of the fact that both series of compounds are isoelectronic. [1] More importantly these dissimilarities cannot be easily explained in terms of the atoms in molecules (AIM) theory or in terms of the population of the πBN* or πCC* antibonding orbitals. In some molecules a decrease (increase) of the electron density is unexpectedly reflected in a shortening (lengthening) of the bond. Conversely, the ELF analysis offers a clear picture of the substituent effects on the CC and BN bonding. The dissimilarities between acetylene- and iminoborane derivatives are primarily a consequence of the strong electronegativity difference between the B and N atoms in the iminoboranes. This difference is the origin of the significant distortion of the BN bonding electron density in iminoboranes, which is not seen in the corresponding acetylene analogues.
References
[1] O. Mó; M. Yáñez; A. Martín Pendás; J.E. Del Bene; I. Alkorta and J. Elguero, Phys. Chem. Chem. Phys. 9, 3970 (2007)
P. J. MacDougall
Address
Title of the talk
Abstract...
References
[1]
[2]
J. Angyan
Address
Title of the talk
Abstract...
References
[1]
[2]
M. Causa
Address
Title of the talk
Abstract...
References
[1]
[2]
J. Contreras-Garcia
Address
Title of the talk
Abstract...
References
[1]
[2]
D. L. Cooper
Address
Title of the talk
Abstract...
References
[1]
[2]
A. Lüchow
Address
Title of the talk
Abstract...
References
[1]
[2]
E. P. Fowe
Address
Title of the talk
Abstract...
References
[1]
[2]
S. Grabowski
Address
Title of the talk
Abstract...
References
[1]
[2]
A. M. Pendas
Address
Title of the talk
Abstract...
References
[1]
[2]
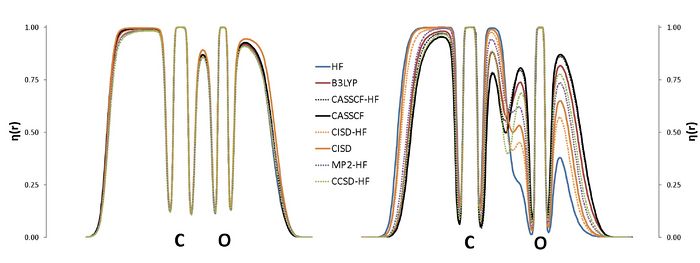
E. Matito
Institute of Physics, University of Szczecin, Poland
The Electron Localization Function at the Correlated Level: A Natural Orbital Formulation.
The ELF is nowadays used in a wide context, with applications on aromaticity, chemical bonding or reaction mechanisms. The calculation of the ELF involves the computation of the second-order density matrix (DM2),1 which has a very simple structure in the case of monodeterminantal wavefunctions. In the case of correlated wavefunctions, the structure of the DM2 imposes an important bottleneck for both the calculation of the ELF and the population analysis (variance, covariance) of the ELF basins. Therefore, in practice, the use of the ELF for correlated wavefunctions is limited to very small molecules. However, it is possible to lower the numerical complexity of the calculation of the localization function as well as that of the pair populations using approximated expressions for the calculation of DM2. In this work we propose a correlated version of the ELF where the DM2 has been approximated by the formula for the monodeterminantal wavefunctions (X-HF in the graph below) and the formula of Müller in terms of natural orbitals.3,4 The approximation has been tested in a set of molecules, NH3, CO2, H2O, CO, CN-, NO+, N2, H2O2, F2 and FCl-, which have been calculated using a plethora of methods: HF, B3LYP, MP2, CISD, CCSD and CASSCF. The present approach aims at a correlated version of the ELF which can be used in medium size molecules.
References
[1] Matito E., Silvi B., Duran M. and Solà M., J. Chem. Phys. 125, 024301 (2006)
[2] Feixas F., Matito E., Duran M., Solà M. and Silvi B., in preparation.
[3] Müller A.M.K., Phys. Lett. 105A, 446 (1984)
[4] M. A. Buijse, E. J. Baerends, Mol. Phys., 100, 401 (2002)
D. Borgis
Address
Title of the talk
Abstract...
References
[1]
[2]
R. Vuillemier
Address
Title of the talk
Abstract...
References
[1]
[2]
J. Cioslowski
Address
Title of the talk
Abstract...
References
[1]
[2]
J. Pilmé
Address
Title of the talk
Abstract...
References
[1]
[2]
R. Nesper
Address
Title of the talk
Abstract...
References
[1]
[2]
Y. Grin
Address
Title of the talk
Abstract...
References
[1]
[2]
J-F. Halet
Address
Title of the talk
Abstract...
References
[1]
[2]
U. Wedig
Address
Title of the talk
Abstract...
References
[1]
[2]
R. Weihrich
Address
Title of the talk
Abstract...
References
[1]
[2]
P. Raybaud
Address
Title of the talk
Abstract...
References
[1]
[2]
E. Dumont
Address
Title of the talk
Abstract...
References
[1]
[2]
N. Chéron
Address
Title of the talk
Abstract...
References
[1]
[2]
S. Berski
Address
Title of the talk
Abstract...
References
[1]
[2]
L. Joubert
Address
Title of the talk
Abstract...
References
[1]
[2]
H. Bolvin
Address
Title of the talk
Abstract...
References
[1]
[2]
A. Delalande
Address
Title of the talk
Abstract...
References
[1]
[2]
R. Nalewajski
Address
Title of the talk
Abstract...
References
[1]
[2]
A. Scemama
Address
Title of the talk
Abstract...
References
[1]
[2]
J. Tao
Address
Title of the talk
Abstract...
References
[1]
[2]
I. Fourré
Address
Title of the talk
Abstract...
References
[1]
[2]
H. Gérard
Address
Title of the talk
Abstract...
References
[1]
[2]
N. Russo
Address
Title of the talk
Abstract...
References
[1]
[2]