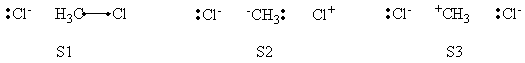Différences entre les versions de « VBTutorial3 »
| Ligne 333 : | Ligne 333 : | ||
| | | | ||
== Exercise 2 : computation of H—H + H. -> H. + H—H radical exchange VBSCD diagram == | == Exercise 2 : computation of H—H + H. -> H. + H—H radical exchange VBSCD diagram == | ||
| − | |||
===1/ Paper exercise :=== | ===1/ Paper exercise :=== | ||
| Ligne 362 : | Ligne 361 : | ||
c/ It is known that for strong binders, at any given bonding distance the singlet-triplet transition energy is larger than twice the bonding energy of the dimer at equilibrium distance, so that one can write the approximate expression <big> <math>\Delta E_{ST} </math>' <math> = 2 BDE </math></big>, where <big><math> BDE </math></big> is the bonding energy of the dimer at equilibrium distance. Using the latter expression, express the avoided crossing term <big><math> B </math></big> as a function of the bonding energy of <big><math> H_{2}</math></big>. | c/ It is known that for strong binders, at any given bonding distance the singlet-triplet transition energy is larger than twice the bonding energy of the dimer at equilibrium distance, so that one can write the approximate expression <big> <math>\Delta E_{ST} </math>' <math> = 2 BDE </math></big>, where <big><math> BDE </math></big> is the bonding energy of the dimer at equilibrium distance. Using the latter expression, express the avoided crossing term <big><math> B </math></big> as a function of the bonding energy of <big><math> H_{2}</math></big>. | ||
| + | {| class="collapsible collapsed wikitable" | ||
| + | |- | ||
| + | !'''Answer''' | ||
| + | |- | ||
| + | | | ||
| + | a - Considering the R and R* states for the radical exchange process, the HL wave functions for R and R* are the following :<br> | ||
| + | <math>\psi (R)=(|xa\overline{y}|-|x\overline{a}y|)(1/2)^{-1/2} </math><br> | ||
| + | <math>\psi (R^{*})=(|x\overline{a}y|-|\overline{x}ay|)(1/2)^{-1/2}</math><br> | ||
| + | At the equilibrium distance of R, A and Y are close together while X is far away. Therefore R is stabilized by a covalent bond and its energy is <big><math> 2 \beta S </math></big>. In R*, the AY entity has two parallel spins 50% of the time (a repulsive situation), and alternated spins 50% of the time (a non-bonding situation). Therefore the energy of R* is half a triplet repulsion, i.e. <big><math> - \beta S </math></big>. As <big><math>\Delta E_{ST} (A-Y)= 4 \beta S </math></big>, the final G expression is:<br> | ||
| + | <big><math>G=0.75 \Delta E_{ST} (A-Y)</math></big><br> | ||
| + | |||
| + | b - Let us express the various energies and matrix elements in terms of the usual <big><math> \beta </math></big> and <big><math> S </math></big> integrals between the X and H orbitals: | ||
| + | |||
| + | <big><math> E_{ind} = 2 \beta S </math></big> | ||
| + | |||
| + | <big><math> S_{12} = 0.5 </math></big> | ||
| + | |||
| + | <big><math> H_{12} = 2 \beta S </math></big> | ||
| + | |||
| + | <big><math> RE = (2 \beta S - 0.5 \beta S )/1.5 = \beta S </math></big> | ||
| + | |||
| + | Since at the transition state geometry <big> <math>\Delta E_{ST} </math>'</big> = <big><math> 4 \beta S </math></big>, we have: | ||
| + | |||
| + | <big> <math>B = 0.25\Delta E_{ST} </math>'</big> | ||
| + | |||
| + | c - Since <big> <math>\Delta E_{ST} </math>' <math> = 2 BDE </math></big>, we may re-express the avoided crossing term as: | ||
| + | |||
| + | <big> <math>B = 0.5BDE </math></big> | ||
| + | |} | ||
=== 2/ Computer exercise === | === 2/ Computer exercise === | ||
Version du 12 juillet 2012 à 12:00
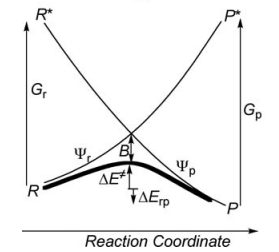
Valence Bond State correlation diagrams
Exercise 1 : Computation of state correlation Diagrams for a 3 centers / 4 electrons system
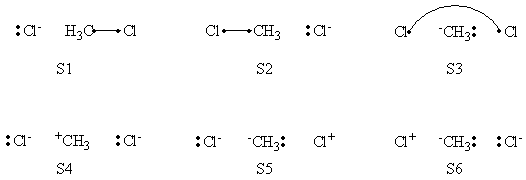
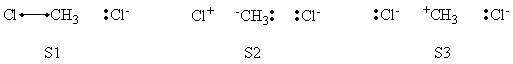
In this exercise the <math>\textrm{S}_{\textrm{N}}2</math> reaction Cl<math>{}^{-}</math> + CH3Cl -> ClCH3 + Cl<math>{}^{-}</math> will be studied in both vacuum and solution. Valence Bond State Correlation Diagrams (VBSCD) will be constructed at <math>\pi</math>-D-BOVB level. There are two parts in this exercise: basic part and optional part. The basic part is performed with MCP-DZP basis set in which the inner orbitals in Cl and C are described with MCP pseudo potential. The optional part is performed with 6-31+G* basis set, using the general specification for the xmvb input (expert users). Only reactant and transition state will be computed in this exercise, which is sufficient to build the VBSCD diagrams.
| Note:How to perform a VBPCM calculation |
|---|
|
A VBPCM calculation is performed in the similar way as the VB calculations in vacuum. One should prepare a GAMESS input file with solvent assigned such as: The details of PCM calculation in the GAMESS can be found in GAMESS manual. Keyword "VBTYP=XMVB" in CONTRL section is also essential. After the GAMESS input file is prepared, an XMI file with keyword "VBPCM" should be prepared with the same file name as GAMESS input file. In the current XMVB package, VBSCF/PCM and BOVB/PCM calculations are both supported. |
| Basic part |
|---|
1. Compute the Energies and Wavefunctions at Reactant and Transition State with Different Sets of VB Structures
2. Analysis: Wavefunctions and Energies
|
| Optional part (expert users) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In this part, calculations with BFI section are performed with 6-31+G* basis set, which is desired for the experienced users. The inner orbitals are frozen as HF orbitals in all VB calculations and the valence basis functions are reorganized to hybrid basis functions so that the <math>\sigma</math>, <math>\pi_x</math> and <math>\pi_y</math> spaces can be separated well. A D-BOVB calculation is performed in 2 steps:
The VB calculations are the same as the calculations performed above. Try to understand the BFI section, perform the calculations and compare the differences of barrier heights, resonance energies and performances with and without $BFI.
|
>> general guidelines for BOVB calculations
| Optional exercises - Homework | ||
|---|---|---|
Exercise 2 : computation of H—H + H. -> H. + H—H radical exchange VBSCD diagram1/ Paper exercise :a/ Considering the following radical exchange process: <math> X^{\bullet} + A-Y \rightarrow X-A + ^{\bullet}Y </math> (X = A = Y = hydrogen atom) Write the HL wave functions for R and R* and derive the value of G using semiempirical VB theory. Hints : 1) write the wave functions of R and R* so that their overlap is positive; 2) neglect the overlap beween the external atoms X and Y, and neglect the overlap between two different determinants. b/ Considering the following reaction:
<math>
X^{\bullet} + H-X \rightarrow X-H +^{\bullet}X
</math> <math>RE = [H_{12}-E_{ind}S_{12}]/(1+S_{12}) </math> where <math> E_{ind} </math> is the energy of an individual VB structure, and <math> S_{12} </math> and <math> H_{12} </math> are respectively the overlap and Hamiltonian matrix element between R and R*. c/ It is known that for strong binders, at any given bonding distance the singlet-triplet transition energy is larger than twice the bonding energy of the dimer at equilibrium distance, so that one can write the approximate expression <math>\Delta E_{ST} </math>' <math> = 2 BDE </math>, where <math> BDE </math> is the bonding energy of the dimer at equilibrium distance. Using the latter expression, express the avoided crossing term <math> B </math> as a function of the bonding energy of <math> H_{2}</math>.
2/ Computer exerciseVBSCD for H—H + H. -> H. + H—H at VBSCF then VBCISD level. In this exercise the VBSCD for H—H + H. -> H. + H—H at VBSCF then VBCISD level will be computed with 6-31G**. Computations for reactant and transition state are requested and other points are optional for advanced users.
Exercise 3 (paper exercise) : Conical intersection in H3• radical(for further reading, see S. Shaik and P.C. Hiberty, "The Chemist's Guide to VB theory", Wiley, Hoboken, New Jersey, 2008, pp. 157-161, exercises 6.11-6.14 pp. 174-176, and answers to the exercises pp. 188-192. Consider three hydrogen atoms Ha, Hb, Hc, with respective atomic orbitals a, b and c, and the two VB structures The Ha-Hb and Hb-Hc distances are equal.
Appendix : Thumb rules for the calculations of effective Hamiltonian matrix elements between determinants.
<math> <D|H|D'>=<|...i\overline{j}...||H||...\overline{i}j...|>= -2 \beta_{ij} S_{ij}</math> (for <math>D</math>, <math>D'</math> differing by spin inversion of two spin-orbitals) |