The SD BOVB method
Révision datée du 5 juillet 2012 à 11:14 par Benoit (discussion | contributions)
<<< Back to the "general guidelines for BOVB calculations"
Definition of the S-BOVB level
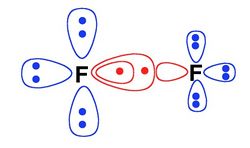
The "S" in the name means "splitted". At this level, in each VB structure, all the active orbitals which are doubly occupied at the L-BOVB level are "splitted", that is to say they are replaced by a pair of singlet-coupled electrons in two different orbitals located on the same center. Here is below a pictorial example for the F-F+ ionic structure of difluorine dimer. This allows the inclusion of some more dynamical correlation (radial correlation) for the active electron pairs.