General guidelines for BOVB calculations
Active space
First, choose an active system of orbitals/electrons in the molecule under study. This is the part of the molecule that will be treated in a VB way : for example the bonds that are made/broken in a reaction, or the orbitals whose occupancy varies in a series of resonating VB structures. The occupancies of the active orbitals will change from one VB structure to the other. The other orbitals, called « inactive » or « spectator », are those that keep the same occupancy in all VB structures.
BOVB levels
Each active orbital must be defined as localized, either on a single atom or on a fragment (recommended, see next section below). The inactive orbitals may be kept localized (L-VBSCF or L-BOVB) or allowed to delocalize on the whole molecule (D-VBSCF or D-BOVB). Better accuracy is obtained in this latter case. Last, in the ionic structures the doubly occupied active orbitals may be "splitted" for a better description, leading to the S-BOVB (if inactive are kept localized) and SD-BOVB levels.
Recommended definition for the orbital blocks
These rules lead to the definition of "orbital blocks" :
- From your active orbitals divide the molecule into fragments, such as two active orbitals involved in a covalent coupling in one of the structures belong to different fragments;
- To each fragment is associated a localization space : the orbitals belonging to a specific localization space can only span the basis functions centered on atoms belonging to this fragment;
- The different localization spaces may then be further divided according to the symmetry of the molecule (<math>\sigma</math>/<math>\pi</math> separation for instance), which provides the different orbital blocks.
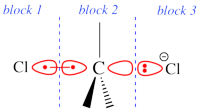
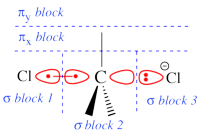
Two example choices of orbital blocks definitions for Cl-(Me3)C-Cl- SN2 transition state. Note that the r.h.s. choice corresponds to the so-called π-D-VBSCF and π-D-BOVB levels (see also example 2 below), and that they won't be valid for reactant and product geometries where there is no formal <math>\sigma</math> / <math>\pi</math> separation.
| Remarks : |
|---|
Of course, it is not always reasonable to strictly follow this definition for orbital blocks, as it cause in some cases some important physical interactions described by some inactive orbitals to be broken (for instance, if it leads to break a bond described by an inactive MO). In such cases, you may need to define different orbital blocks for inactive and active orbitals which will share some basis functions in common. This might lead in some cases to convergence issues or instabilities (orbital flipping for instance). |
| Exemples : |
|---|
|
Procedure for doing a D-BOVB calculation
General case (low symmetry):
- Perform a ("L") VBSCF calculation together with the keyword boys in the $orb section ;
- perform the L-BOVB calculation, always starting from converged VBSCF orbitals ;
- then perform the D-BOVB calculation : starting from the converged L-BOVB orbital as guess, run the calculation by optimizing the delocalized inactive orbitals, while freezing the active orbitals during the optimization (put "0" as coefficient in first $orb line).
High symmetry case:
This is even simpler. This case is encoutered when the inactive orbitals that we wish to delocalize can be distinguished from the active orbitals by symmetry. Example : we wish to delocalize the inactive π orbitals of F2, while the active orbitals are of σ type. In such a case, one may let the VBSCF wave function converge directly in a single step (called D-VBSCF), by defining the π orbitals as delocalized, and the active orbitals and remaining inactive orbitals as localized with σ symmetry. Note that in this case the σ inactive lone pairs of F2 will not be delocalized. Since the delocalization of inactive orbital is not complete, we may refer this calculation as π-D-VBSCF. The π-D-BOVB is got the same way, taking the as D-VBSCF as a guess.
| Remark : |
|---|
|
In the particular case where all orbital blocks contain only either active or inactive orbitals, all blocks containing inactive orbitals could be grouped into a single one, and then one works at the "D" level from the start (D-VBSCF, then D-BOVB calculation). This is the case for instance when there is a <math>\sigma</math>/<math>\pi</math> separation in your molecule with all (and only) <math>\pi</math> pairs taken as active (see tutorial 2 exercises). |
>> SD-BOVB calculations (advanced user)
General advices
- Do not use diffuse functions unless you deal with an anion, and do not use larger than triple-zeta basis sets
- Impose a high molecular symmetry if possible (case of a distorted molecule which slightly departs from a higher symmetry point group) ;
- Use orbtyp=hao together with fragtyp=sao as soon as you have some <math>\sigma</math>/<math>\pi</math> symmetry in your molecule,
- In the $ctrl section of the XMVB input, you should use the "iscf=5" algorithm for VBSCF calculations, and change it to "iscf=2" for BOVB calculations.
- Use the boys keyword at the VBSCF step, as it provides more physically meaningful orbitals for the subsequent BOVB calculations (this is particularly important if you want to go up to the S- or SD-BOVB levels)
- Always use a set of converged orbitals from the preceding step, ex : use converged VBSCF orbitals to start a L-BOVB calculation, converged L-BOVB to start a S-BOVB or a D-BOVB calculation, and converged S-BOVB orbitals to start a SD-BOVB calculation.
| Convergence issues |
|---|
|
Sometimes, BOVB calculations are subject to instabilities that it is important to detect. They may manifest as incredibly high bonding energies, generally associated with strongly negative weights of the VB structures according to the Choiirgwin-Coulson definition. Then, how I know whether or not my BOVB calculation went well ? Check the following quantities :
What can I do if I encounter an instability or convergence issue ? Check the following points :
How to cure the problem - if I haven't done any particular "mistake" :
|