VBTutorial3
How to modify this page :
- first : log in (top right of this page) ;
- click on [edit] (far right) to edit a section of the page ;
- write your text directly in the wiki page, and click on the "Save page" button (bottom left) to save your modifications
Pictures : how to insert a picture in your text
See also this page for an introduction to the basics of the wiki syntax
Remarks
Exercises
Main Exercise
Exercise 1 : Computation of state correlation Diagrams for a 3 centers / 4 electrons system
- Computer exercise :
In this exercise the <math>\textrm{S}_{\textrm{N}}2</math> reaction Cl<math>{}^{-}</math> + CH3Cl -> ClCH3 + Cl<math>{}^{-}</math> will be computed in both vacuum and solution, at <math>\pi</math>-D-BOVB level. The basis set is 6-31+G*, for there is an anoin in the system. To make the computation simplified, only reactant and transition state will be computed in the exercise. It is recommended to the advanced users to draw the whole VBSCD.
Compute the Energies and Wavefunctions at Reactant and Transition State with Different Set of VB Structures
- Write the following VB structure sets, compare and see the differences:
- all structures;
- minimal structures for reactant;
- minimal structures for product.
- Perform BOVB calculation for reactant:
- Perform VBSCF calculation for reactant at VBSCF level, with delocalized <math>\pi</math>-spaces, localized <math>\sigma</math>-space and all VB structures;
- Perform BOVB calculation for reactant at BOVB level, with VBSCF orbital as initial guess. The VB structures and orbital descriptions are the same as at VBSCF level.
- Do the same BOVB calculations with minimal structures for reactant and product.
- Perform BOVB calculation for transition state. The procedure is the same as step 2.
- Perform BOVB/PCM calculations for reactant:
- Perform VBSCF/PCM calculation for reactant, with VBSCF orbitals as initial guess.
- Do the BOVB/PCM calculation for reactant with VBSCF/PCM orbitals as initial guess.
- Do the BOVB/PCM calculations with minimal structures for reactant and product.
- Perform BOVB/PCM calculations for transition state with the same procedure as in step 4.
Analyze the Data from Above Computations
- Compute the Barrier height of the <math>\textrm{S}_{\textrm{N}}2</math> reaction in both vacuum and solution. See the difference of the barrier heights, and find out the reason.
- Compare the energies of reactant and product structures at reactant and transition state, in both vacuum and solution. What's the difference of the energies at different points? Why?
- Compute the resonance energies at both reactant and transition state points, see the difference of the resonance energies.
- Compute VBSCD diagrams for Cl(-) + CH3Cl -> ClCH3 + Cl(-), at π-D-BOVB levels, first in gas phase then using VB(PCM)... Which basis set should we use : 6-31+G*. As this is an anion we should add a set of diffuse functions, but then there may be trouble with BOVB... Check first that everything is fine at BOVB level (no instability)...
- Reactant and product geometry will be CH3Cl and Cl(-) computed separately (CH3Cl has already been computed in tutorial 1, we just have to change the basis set, which is easy with XMVB 2.0 !) ;
- TS transition state : we take the linear TS, with orientation of the x or y axis along a C-H bond this will make a <math>\sigma</math>, <math>\pi_x</math>, <math>\pi_y</math> separation, so we could apply π-D-BOVB level, localizing all <math>\sigma</math> pairs (including inactive <math>\sigma</math> lone pairs and cores of Carbon and Chlorine atoms, and delocalizing the and <math>\pi_x</math> and <math>\pi_y</math> inactive orbitals from the beginning ((see also "high symmetry cases" here)|.
>> general guidelines for BOVB calculations
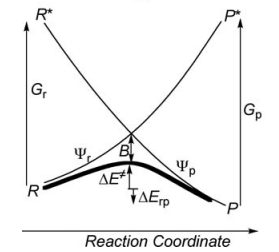
Exercise 2 : computation of H—H + H. -> H. + H—H radical exchange VBSCD diagram
1/ Paper exercise :
a/ Considering the following radical exchange process: <math> X^{\bullet} + A-Y \rightarrow X-A + ^{\bullet}Y </math> (X = A = Y = hydrogen atom)
Write the HL wave functions for R and R* and derive the value of G using semiempirical VB theory. Hints : 1) write the wave functions of R and R* so that their overlap is positive; 2) neglect the overlap beween the external atoms X and Y, and neglect the overlap between two different determinants.
b/ Considering the following reaction:
<math>
X^{\bullet} + H-X \rightarrow X-H +^{\bullet}X
</math>
Use semiempirical VB theory to derive the following expression for the avoided crossing term <math> B </math> :
<math>B = 0.25\Delta E_{ST} </math>'
where <math>\Delta E_{ST} </math>' is the singlet-triplet transition energy of the X-H bond at the geometry of the transitions state.
Indication: the resonance energy arising from the mixing of two degenerate VB structures is given by the following expression:
<math>RE = [H_{12}-E_{ind}S_{12}]/(1+S_{12}) </math>
where <math> E_{ind} </math> is the energy of an individual VB structure, and <math> S_{12} </math> and <math> H_{12} </math> are respectively the overlap and Hamiltonian matrix element between R and R*.
c/ It is known that for strong binders, at any given bonding distance the singlet-triplet transition energy is larger than twice the bonding energy of the dimer at equilibrium distance, so that one can write the approximate expression <math>\Delta E_{ST} </math>' <math> = 2 BDE </math>, where <math> BDE </math> is the bonding energy of the dimer at equilibrium distance. Using the latter expression, express the avoided crossing term <math> B </math> as a function of the bonding energy of <math> H_{2}</math>.
2/ Computer exercise :VBSCD for H—H + H. -> H. + H—H at VBSCF then VBCISD level.
In this exercise the VBSCD for H—H + H. -> H. + H—H at VBSCF then VBCISD level will be computed with 6-31G**. Computations for reactant and transition state are requested and other points are optional for advanced users.
Compute the Energies and Wavefunctions at Reactant and Transition State with Different Set of VB Structures
- Write the following VB structure sets, compare and see the difference, and think about why:
- all structures;
- minimal structures for reactant;
- minimal structures for product.
- Compute reactant at VBSCF and VBCISD level:
- Compute reactant at VBSCF level with "orbtyp=hao" and "boys";
- Compute reactant at VBCISD level, with VBSCF orbital as initial guess.
- Do the same to computations with minimal structures for reactant and product point.
- Compute transition state at VBCISD level with the same procedure as for reactant.
Analyze the Data from Above Computations at VBSCF and VBCISD levels
- Compute the Barrier height of the <math>\textrm{S}_{\textrm{N}}2</math> reaction at VBSCF and VBCISD levels. See the difference of the barrier heights.
- Compare the energies for reactant and product structures at reactant and transition state, by both VBSCF and VBCISD. What's the difference of the energies at different points? Why?
- Compute the resonance energies at both reactant and transition state points, see the difference of the resonance energies.
Optional: Compute all points and draw the VBSCDs at VBSCF and VBCISD levels.
Exercise 3 (paper exercise) : Conical intersection in H3• radical
(for further reading, see S. Shaik and P.C. Hiberty, "The Chemist's Guide to VB theory", Wiley, Hoboken, New Jersey, 2008, pp. 157-161, exercises 6.11-6.14 pp. 174-176, and answers to the exercises pp. 188-192.
Consider three hydrogen atoms Ha, Hb, Hc, with respective atomic orbitals a, b and c, and the two VB structures ![]() ] and
] and ![]() ] .
] .
The Ha-Hb and Hb-Hc distances are equal. ![]()
- By using the thumb rules recalled below, where squared overlap terms are neglected, derive the expression of the energies of R and P, and of the reduced Hamiltonian matrix element between R and P for the 3-orbital/3-electrons reacting system [Ha--Hb--Hc]•.
- From the sign of this latter integral when θ > 60°, derive the expressions of the ground state Ψ≠ and of the first excited state Ψ* of the H3• system. One may drop the normalization constants for simplicity. What bonding scheme does the excited state represent ?
- Show that the reduced Hamiltonian matrix element is largest in the collinear transition state geometry, and drops to zero in the equilateral triangular structure.
- Show that R and P VB structures are degenerate in the equilateral triangular structure, and that Ψ≠ and Ψ* are also degenerate in this geometry.
- We now extend the above conclusions to the allyl radical. What are the bonding schemes corresponding to the ground state and first excited state ? What geometrical distortion would make these two states degenerate ? What would be the end product of a photochemical excitation of allyl radical to its first excited state ?
Appendix : Thumb rules for the calculations of effective Hamiltonian matrix elements between determinants.