Abstracts of the TCTC 2014
Slides available after the workshop
HOW TO UPLOAD YOUR ABSTRACT
In order to upload your abstract, you will need a valid login account.
Please contact us email and we will email you your login details.
Please be aware that the whole system has been reinitialized, so old accounts are not valid anymore.
CONTRIBUTORS: please add below, in your own section, your title talk and abstract :
- first : log in;
- click on your name in the "Contents" box below, this will lead you to your own section;
- your section starts with your name as the title line, click on [edit] (far right).
- >>> How to insert a picture in your abstract
- SPEAKERS: A template has been created with you name for you to fill in. Please upload your abstracts before 31st March 2014.
- POSTERS: If you wish to contributethe with a poster, feel free to follow the prescribed template in the Poster section . Please upload your abstracts before 30th June 2014.
This is an example
Contributor's name
Affiliation
Title
Summary
[1] Popelier, P. L. A.; Brémond, É. A. G. Int.J.Quant.Chem. 2009, 109, 2542.
High accuracy methods /Relativistic corrections
Seiichiro Ten-no
Department of Computational Science, Graduate School of System Informatics, Kobe University
Recent advances in explicitly correlated electronic structure theory
In quantum chemistry, one of the main obstacles to accurate electronic structure calculations is the slow convergence of a CI expansion. F12 theory exploiting geminal basis functions with the Slater correlation factor leads to greatly improved convergence of correlation energies. Recent developments based on the rational generator approach from the cusp conditions is summarized. Four-component relativistic treatment and massively parallel implementation of F12 methods will be also presented.
Tron Saue
Laboratoire de Chimie et Physique Quantiques, Université de Toulouse 3 (Paul Sabatier), 118 route de Narbonne, 31062 Toulouse, France
Parity violation in chiral molecules
The parity violation associated with the weak force implies that left- and right-hand forms of chiral molecules are diastereomers rather than enantiomers. A predominantly French collaboration of theoreticians and experimentalists, physicists and chemists, hunt for the signature of parity violation in the vibrational spectra of chiral molecules. The task of theory, using relativistic molecular quantum chemistry, is to guide experiment in the search for suitable candidate molecule.
In this talk I review recent progress with particular emphasis on the analysis and understanding of the mechanism of parity violation in molecules. A successful experiment will have a significant impact on our understanding of the stability and dynamics of chiral molecules as well as the origin of biochirality.
[1] Radovan Bast, Anton Koers, André Severo Pereira Gomes, Miroslav Ilia², Lucas Visscher, Peter Schwerdtfeger and Trond Saue, Analysis of parity violation in chiral molecules, Phys. Chem. Chem. Phys. 13 (2011) 854
[2] Benoit Darquié, Clara Stoeffer, Alexander Shelkovnikov, Christophe Daussy, Anne Amy-Klein, Christian Chardonnet, Samia Zrig, Laure Guy, Jeanne Crassous, Pascale Soulard, Pierre Asselin, Thérèse R. Huet, Peter Schwerdtfeger, Radovan Bast and Trond Saue, Progress toward the first observation of parity violation in chiral molecules by high-resolution laser spectroscopy, Chirality 22 (2010) 870
Toru Shiozaki
Department of Chemistry, Northwestern University
Modern Multireference Electron Correlation Methods
In this lecture, I will present the state of the art of multireference electron correlation theories for strongly correlated systems. The standard quantum chemistry methods, such as density functional theories and single-reference coupled-cluster theories, break down when wave functions are not approximated well by a single Slater determinant. Such situation includes (1) electronic structures of transition metal complexes that are important for catalytic reactions; (2) two-electron excited states that play a central role in next-generation solar energy conversion; and (3) state-crossing between the ground and excited states, relevant to photo-switches. First, I will describe the so-called completely active space self-consistent field (CASSCF) method that is a multi-determinant extension of the Hartree–Fock method. I will also discuss various approximate variants of CASSCF, including our recent development of an active space decomposition strategy and its connection to density matrix renormalization group. Second, I will present conventional electron correlation models that are based on multi-determinant reference states, such as second-order perturbation (CASPT2), configuration interaction (MRCI), and so on. Finally, I will present new approaches in this field, such as integration with explicit correlation methods, multireference coupled cluster methods, and beyond.
Density functional theory
Guanhua Chen
Affiliation
Title
Summary
Xin Xu
Collaborative Innovation Center of Chemistry for Energy Materials, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, MOE Laboratory for Computational Physical Science, Department of Chemistry, Fudan University, Shanghai, 200433, China
Fractional Charge Behaviour and Band Gap Predictions with the XYG3 Type of Doubly Hybrid Density Functionals
Abstract
There remain major challenges in using density functional theory (DFT) to accurately predict some important chemical properties such as ionization potential (IP), electron affinity (EA) and their difference (i.e. fundamental band gap, Eg = IP – EA).[1] The main problem of relating Kohn-Sham (KS) frontier orbital energies to these properties have been traced to the delocalization errors by Yang and co-workers,[2] because common approximate functionals exhibit a convex behaviour in violation of the exact linearity condition for fractional charges. It is not clear, however, how a new class of approximate functionals, namely doubly hybrid (DH) functionals, behaves in this situation. We present here such an analysis and show that the XYG3 type of DH functionals give good agreement between the frontier orbital energies and the experimental IP, EA, and Eg, as expected from their nearly straight line fractional charge behaviors.[3] the
Acknowledgments: Financial supports from the National Natural Science Foundation of China (91027044, 21133004), and the Ministry of Science and Technology (2013CB834606, 2011CB808505) are gratefully appreciated.
References: [1] J.P. Perdew, R.G. Parr, M. Levy, J.L. Balduz,Jr. Phys. Rev. Lett. 1982, 49, 1691. [2] A.J. Cohen, P. Mori-Sánchez and W.T. Yang, J. Chem. Theory Phys. 2009, 5, 786. [3] N.Q. Su, W.T. Yang, P. Mori-Sánchez, X. Xu, J. Phys. Chem. A, 2014, DOI:10.1021/jp5029992.
Weitao Yang
Department of Chemistry and Physics, Duke University, Durham, N.C. 27708, U.S.A.
Exchange-Correlation and Electronic Excitation Energies from Pairing Matrix Fluctuations
Abstract
We have developed an adiabaIn the first part of this talk, I will describe the extension of the density matrix embedding method to obtain arbitrary frequency-dependent correlation functions. This is achieved via the embedding of a local response function within a frequency dependent set of bath states. These couple the excitations in the local region to the entire delocalized bandstructure of the material, and can converge dynamic quantities to the thermodynamic limit. tic connection to formulate the ground-state exchange-correlation energy in terms of pairing matrix fluctuations. This formulation of the exchange-correlation energy opens new a channel for density functional approximations based on the many-body perturbation theory. We illustrate the potential of such approaches with an approximation based on the particle-particle Random Phase Approximation (pp-RPA). This resulting method has many highly desirable properties. It has minimal delocalization error with a nearly linear energy behavior for systems with fractional charges, describes van der Waals interactions similarly and thermodynamic properties significantly better than the conventional RPA, and eliminates static correlation error for single bond systems. Most significantly, it is the first known functional with an explicit and closed-form dependence on the occupied and unoccupied orbitals, which captures the energy derivative discontinuity in strongly correlated systems.
We also adopted pp-RPA and the particle-particle Tamm-Dancoff approximation (pp-TDA) to approximate the pairing matrix fluctuation and then determine excitation energies by the differences of two-electron addition/removal energies. This approach captures all types of interesting excitations: single and double excitations are described accurately, Rydberg excitations are in good agreement with experimental data and CT excitations display correct 1/R dependence. Furthermore, the pp-RPA and the pp-TDA have a computational cost similar to TDDFT and consequently are promising for practical calculations.
To further explore the potential use of pairing matrix dependent functionals, we developed the linear-response time-dependent density-functional theory with pairing fields with both adiabatic and frequency-dependent kernels. The linear-response theory is established based on the representability assumption of the pairing matrix. The linear response theory justifies the use of approximated density functionals in the pp-RPA equation. This work sets the fundamentals for future density-functional development to enhance the description of ground state correlation energies and N ± 2 excitation energies.
References:
1. J. Cohen, P. Mori-Sanchez, and W. T. Yang. Challenges for Density Functional Theory. Chem. Rev. 112:289, 2012
2. H. van Aggelen, Y. Yang and W. T. Yang. Exchange-correlation energy from pairing matrix fluctuation and the particle-particle random-phase approximation, PHYSICAL REVIEW A 88, 030501(R), 2013.
3. Y. Yang, H. van Aggelen, S. N. Steinmann, D. Peng, and W. T. Yang. Benchmark tests and spin adaptation for the particle-particle random phase approximation. Journal of Chemical Physics, 139:174110, 2013.
4. D. G. Peng, S. N. Steinmann, H. van Aggelen, and W. T. Yang. Equivalence of particle-particle random phase approximation correlation energy and ladder-coupled-cluster doubles. Journal of Chemical Physics, 139(10):104112, Sep 2013.
5. Y. Yang, H. van Aggelen, and W. T. Yang. Double, Rydberg and charge transfer excitations from pairing matrix fluctuation and particle-particle random phase approximation. Journal of Chemical Physics, 139, 224105, 2013.
6. D. G. Peng, H. van Aggelen, Y. Yang and W. T. Yang. Linear-response time-dependent density-functional theory with pairing fields. Journal of Chemical Physics, 140, 18A522, 2014.
Thomas Frauenheim
Affiliation
Title
Summary
Mario Piris
Kimika Fakultatea, Euskal Herriko Unibertsitatea (UPV/EHU); Donostia International Physics Center (DIPC); IKERBASQUE, Basque Foundation for Science
NOF theory as an alternative to DFT
In 1974, Gilbert proved for the one-particle reduced density matrix (1-RDM) an analogous theorem to the Hohenberg-Kohn theorem for the density. Accordingly, one can employ the exact energy functional, with an approximate 2-RDM that is built from the 1-RDM using a reconstruction functional, to describe a molecule. The major advantage of a 1-RDM formulation is that the kinetic energy is explicitly constructed and does not require a functional. Like for the density, the ensemble N-representability conditions of the 1-RDM are well-known, but this does not overcome the N-representability problem of the energy functional.
The 1-RDM functional is called Natural Orbital Functional (NOF) when it is based upon the spectral expansion of the 1-RDM. An approximate reconstruction, in terms of the diagonal 1-RDM, has been achieved by imposing necessary N-representability conditions on the 2-RDM. Appropriate forms of the two-particle cumulant have led to different implementations, known in the literature as PNOFi (i=1,5) being the most successful the PNOF5, and its extended version PNOF5e. On the other hand, antisymmetrized product of strongly orthogonal geminals (APSG) with the expansion coefficients explicitly expressed by means of the occupation numbers have been used to generate these NOFs, which demonstrates strictly the N- representability, size-extensivity and size-consistency of the functionals. Moreover, it opens the possibility of using a perturbation theory to recover the missing dynamic correlation.
Recently, an interacting-pair model has been proposed to attain a new NOF: PNOF6. The new approach belongs to the JKL-only family of functionals. PNOF6 is superior to its predecessor PNOF5, which is an independent-pair approach. The functional is able to treat properly the static correlation and recover an important part of dynamic correlation, thereby putting together the advantages of the other members of this functional series.
In this presentation, the theory behind these functionls is outlined, and some examples are presented to illustrate their potentiality. The improvement of PNOF6 over PNOF5 is demonstrated by several examples.
Jianwei Sun
Department of Physics, Temple University, Philadelphia, Pennsilvania, 19122, USA
Meta-generalized gradient approximations in density functional theory
One of the challenges in density functional theory (DFT) is to improve the accuracy of its exchange-correlation functional, the only part to be approximated, while maintaining computational efficiency. On the Jacob’s ladder categorizing the approximations based on their inputs to the exchange-correlation energy density, higher rungs are usually built on and thus improve accuracy over lower ones. However, climbing from the lowest three semilocal rungs [local spin density approximation (LSDA), generalized gradient approximation (GGA), and meta-GGA (MGGA)] to the higher fully-nonlocal rungs usually increases the computational cost dramatically. MGGA is the highest and potentially most accurate semilocal rung of the Jacob’s ladder, which includes as an input the kinetic energy density in addition to the electron density and its gradient used in GGAs. We will show that the inclusion of the kinetic energy density enables MGGA to recognize different chemical bonds (covalent, metallic, and even weak ones) and assign different GGA descriptions for different bonds. We further show that better nonlocal functionals can be constructed based on a better semilocal one (e.g., global hybrid MGGA vs. global hybrid GGA).
Frontiers in computation
Robert Harrison and W. Scott Thornton
Institute for Advanced Computational Science, Stony Brook University
Evaluation of the GW method for applications in chemistry
The GW method is one of a hierarchy of many-body methods that forms an
analogue in the theory of solid-state-systems to coupled-cluster family of methods,though it is of necessity based upon the Green’s function rather than the wave
function. We examine the formulation and implementation of the GW and relatedmethods and evaluate their performance in comparison to standard quantum
chemical approaches.
Roland Lindh
Uppsala University
Implementation and Performance of Analytical CD/RI-SA-CASSCF gradients
Authors: Mickaël Delcey, Thomas B. Pedersen, Francesco Aquilante, and Roland Lindh
Abstract: The implementation of analytic nuclear gradients of the state-average Complete Active Space SCF method in the framework of the Resolution-of-Identity approximation and auxiliary basis sets based on Cholesky Decomposition is presented. For optimal scaling the implementation is based on explicit and different treatment of the Coulomb, Exchange and Active parts of the two-particle density matrix. Additionally, the difference between the state-specific and state-average CASSCF implementation, due to the inclusion of the orbital relaxation in the two-particle density matrix, require a none standard approach to preserve the scaling benefits of the RI/CD approach. In this presentation details of the implementations will be reviewed. Examples of applications, in which either the RI/CD approach is instrumental to enable large-basis-set SA-CASSCF calculations or will speed up overall CPU times with up to one order of magnitude as compared to conventional integral treatment (see Figure 1), will be demonstrated.
Kazuo Kitaura
Kobe University, Kobe Japan
The Fragment Molecular Orbital Method and Its Applications to Very Large Molecules
The fragment molecular orbital (FMO) method[1] is an approximate ab initio MO computational method for vary large molecules such as proteins. In the method a molecule is divided into fragments and ab initio MO calculations are performed on the fragments, their dimers and optionally trimers to obtain the total energy and other properties of the whole molecule. The method reproduces regular ab initio properties with good accuracy. Various FMO-based correlation methods have been developed including density functional theory (DFT), 2nd order Møller-Plesset perturbation theory (MP2), coupled cluster theory (CC), and MCSCF. Polarizable continuum model (PCM) was interfaced with FMO, allowing one to treat solvent effects of real size proteins. Recently, the analytical energy gradients and the second derivatives have been developed. In this presentation, I will talk about the FMO method and its applications to biomolecules.
[1] “The Fragment Molecular Orbital Method: Practical Applications to Large Molecular Systems”, Dmitri.G..Fedorov, Kazuo Kitaura, Eds., CRC press, Boca Raton, 2009.
Takahito Nakajima
RIKEN Advanced Institute for Computational Science, Kobe, Japan
NTChem Program Package
An atomic- and molecular-level understanding of drug actions and the mechanisms of a variety of chemical reactions will provide insight for developing new drugs and materials. Although a number of diverse experimental methods have been developed, it still remains difficult to investigate the state of complex molecules and to follow chemical reactions in detail. Therefore, a theoretical molecular science that can predict the properties and functions of matter at the atomic and molecular levels by means of molecular theoretical calculations is keenly awaited as a replacement for experiment. Theoretical molecular science has recently made great strides due to progress in molecular theory and computer development. However, it is still unsatisfactory for practical applications. Consequently, our main goal is to realize an updated theoretical molecular science by developing a molecular theory and calculation methods to handle large complex molecules with high precision under a variety of conditions. To achieve our aim, we have so far developed several methods of calculation. Examples include a way for resolving a significant problem facing conventional methods of calculation, in which the calculation volume increases dramatically when dealing with larger molecules; a way for improving the precision of calculations in molecular simulations; and a way for high-precision calculation of the properties of molecules containing heavy atoms such as metal atoms. We have integrated these calculation methods into a software package named NTChem that we are developing, which can run on the K computer and which contains a variety of high-performance calculation methods and functions. By selecting and combining appropriate methods, researchers can perform calculations suitable for their purpose. For example, it is possible to obtain a rough prediction of the properties of a molecule in a short period of time, or obtain a precise prediction by selecting a longer simulation. In addition, NTChem is designed for high performance on a computer with many compute nodes (high concurrency), and so it makes optimum use of the K computer’s processing power. In this talk, I will introduce the current and future projects for the NTChem software.
Reducing complexity
Garnet Chan
Princeton University, USA
Quantum embeddings
I will give a brief overview of the relationship between dynamical mean-field theory, density matrix embedding, and density functional embedding theory, and describe some recent extensions of density matrix embedding theory to superconductivity, electron-phonon coupling, and quantum dynamics.
Thomas Miller
Caltech, USA
Accurate and systematically improvable density functional theory embedding for correlated wavefunctions
We will describe quantum embedding methods for performing accurate and scalable electronic structure theory calculations in large molecular systems, with application to clusters, liquids, transition metal complexes, and chemical reactions.
[1] "Exact non-additive kinetic potentials for embedded density functional theory." Goodpaster JD, Ananth N, Manby FR, and Miller TF, JCP, 133, 084103 (2010).
[2] "A simple, exact density-functional-theory embedding scheme." Manby FR, Stella M, Goodpaster JD, and Miller TF, JCTC, 8, 2564 (2012).
[3] "Accurate and systematically improvable density functional theory embedding for correlated wavefunctions." Goodpaster JD, Barnes TA, Manby FR, and Miller TF, JCP, 140, 18A507 (2014).
Dominika Zgid
University of Michigan, USA
How to make Dynamical Mean Field Theory quantitative?
In quantum chemistry, calculations for strongly correlated solids are impossible besides the smallest cases because of a prohibitive cost of the explicit treatment of periodic boundary conditions. However, in condensed matter physics, Dynamical Mean Field Theory (DMFT), which is an established method, enables the treatment of solids without explicitly imposing periodic boundary condition. The success of DMFT is based on a self-consistency cycle that takes into account the mutual interaction between a unit cell and the environment. Consequently, one of the most important steps in DMFT is the solution of a Hamiltonian that describes the unit cell and its simplified interaction with the surrounding orbitals. In order to solve such a Hamiltonian, one can use any existing quantum chemistry method that allows us to treat strongly correlated electrons. Such a treatment makes calculations for strongly correlated solids possible. Because of the“frequency”dependence of the embedding, DMFT includes significant correlation effects that go beyond the simpler QM/QM approaches so far used in quantum chemistry. I will introduce our recent contributions in adapting DMFT to quantum chemical calculations and present our latest implementation of the DCA+GF2+DMFT mulitscale hybrid capable of treating weakly and strongly correlated orbitals at the same time. Additionally, I will discuss how the DMFT embedding theory can be developed from a qualitative theory for model systems into a quantitative theory with quantum chemical accuracy.
Gustavo Scuseria
Rice University, USA
Quantum embedding and hierarchical methods
This talk will address two topics currently developed in our research group: quantum embedding and hierarchical methods.
The first topic is based on the DMET proposal of Knizia and Chan (PRL 2013), which we have studied and tweaked [1] in myriad ways. We have recently implemented it for the ab initio Hamiltonian with several impurity solvers including coupled cluster and Projected Hartree-Fock (PHF) theories [2]. Originally designed for strong correlation, DMET works even better for weakly correlated systems. I will present proof-of-principle CC calculations on solids done at a fraction of the computational cost involved in a translationally invariant (k-point integration) calculation.
The second topic is hierarchical symmetry breaking and restoration [3], a natural extension of our PHF work where we treat correlated product states as references instead of single Slater determinants. In this context, the concept of quasi-symmetry appears naturally as a plaquette supersymmetry.
[1] Density matrix embedding theory from broken symmetry mean fields, I. W. Bulik, G. E. Scuseria, and J. Dukelsky, Phys. Rev. B 89, 035140 (2014).
[2] I. W. Bulik and G. E. Scuseria, to be published.
[3] C. A. Jiménez-Hoyos and G. E. Scuseria, to be published.
George Booth
University of Cambridge, UK
Dynamic Correlation Functions in the Condensed Phase
The first part of this talk will describe an extension of the density matrix embedding method to obtain arbitrary frequency-dependent correlation functions. This is achieved via the embedding of a local response function within a frequency dependent set of bath states. These couple the excitations in the local region to the entire delocalized bandstructure of the material, and can converge dynamic quantities to the thermodynamic limit.
This requires the linear response of a correlated set of orbitals, and so the second part of the talk will describe a new approach to obtaining these spectral functions stochastically, in a way which is competitive with the dynamical Lanczos method.
Theoretical spectroscopy / Magnetism
Sonia Coriani
Dipartimento di Scienze Chimiche e Farmaceutiche, Università degli Studi di Trieste, Italy
Modeling the molecular response to electromagnetic fields: challenging spectroscopies and “exotic” effects
Response theory constitutes a powerful and versatile theoretical framework for determining molecular properties and spectra by probing the response to weak (external or internal) electromagnetic fields. It provides efficient computational formulas for both linear and nonlinear response properties, thus describing a plethora of optical effects, and it has become one of the standard tools of computational spectroscopy, contributing to the establishment of the latter as a fundamental player in modern research. Rooted on recent theoretical developments within the coupled-cluster ansatz and density functional theory, in particular so-called “damped” formulations of response theory, aka complex polarization propagator, we present results for both “traditional” and new “perspective” spectroscopies related to the absorption of light by the sample in presence of external or internal magnetic fields, and in the highly energetic x-ray frequency region.
T. Daniel Crawford
Virginia Tech, USA
On the non-locality of higher-order molecular properties: A challenge for reduced-scaling models
The high-degree polynomial scaling of the most reliable quantum chemical models such as coupled cluster theory can be at least partly ameliorated via localized-orbital methods such as the projected-atomic-orbital (PAO) approach, local pair-natural-orbitals (LPNOs), orbital-specific virtuals (OSVs), fragment-molecular orbital (FMO) methods, the incremental scheme, etc. These methods typically yield thermochemical properties with an accuracy comparable to their canonical-orbital counterparts. However, their fidelity for higher-order properties – e.g., those depending on the derivative of the wave function with respect to external electric and magnetic fields – has been explored relatively little. In this talk, we will present our most recent findings in this area, particularly for chiroptical properties such as optical rotations and circular dichroism spectra, the accurate prediction of which is vital for the assignment of absolute sterochemical configurations.
Trygve Helgaker
Centre for Theoretical and Computational Chemistry (CTCC), Department of Chemistry, University of Oslo, Norway
Molecular Magnetism and Magnetic Properties
Molecular magnetism is discussed with emphasis on the calculation of molecular magnetic properties of closed-shell systems by quantum-chemical methods. The importance of gauge invariance is emphasized and illustrated. Recent benchmark results for wave-function and density-functional methods are presented for magnetizabilities, rotational g factors, nuclear shielding constants, spin-rotation constants, and indirect nuclear–nuclear spin-coupling constants. Triplet instabilities and their effect on calculated nuclear spin–spin constants are discussed and illustrated.
Jeppe Olsen
qLeap Centre, Department of Chemistry, University of Aarhus
An Introduction to Molecular Magnetism and Novel Methods using Non-orthogonal Orbitals
The theory of Molecular Magnetism is first briefly described and examples of possible molecular magnets are given. In order to perform wave function calculations on potential molecular magnets it is important to have methods that reliable and accurate can decsribe molecules with many unpaired electrons. A new approach for such calculations are then described. This new approach uses non-orthogonal orbitals, and has a complexity that scales linearly in the number of determinants. This linear scaling is contrast to all previous approaches, where the complexity scales quadratically in the number of determinants. Another feature of the new approach is a direct method for spin-adapting wave functions with many unpaired electrons[1]. As an example of the efficiency of the latter approach, a calculation spin-adapting the Slater determinants of a configuration with 30 unpaired electrons is given. The new method realizes this spin-adaptation in about one minute, whereas the previously used approach is estimated to require seven years of computer time to realize the transformation.
[1] J. Olsen, J. Chem. Phys, 141 , 034112 (2014)
Dage Sundholm
Department of Chemistry, University of Helsinki, Finland
Applications of the gauge-including magnetically-induced current method
The methods of gauge including magnetically induced current method (GIMIC) [1,2,3] will be briefly discussed and an overview of applications of the GIMIC methods will be presented. Recent applications of the GIMIC method include current-density calculations on graphene models, complex multi-ring organic nanorings, porphyrinoids, gaudiene, Möbius twisted molecules, inorganic and all-metal molecular rings, and open-shell species. Studies on hydrogen-bonded molecules indicate that GIMIC can also be used to estimate hydrogen-bond strengths without fragmentation of the system [4].
[1] J. Juselius, J. Gauss, D. Sundholm, J. Chem. Phys. 121 (2004) 3952
[2] S. Taubert, J. Juselius, D. Sundholm, J. Chem. Phys. 134 (2011) 054123
[3] H. Fliegl, S. Taubert, O. Lehtonen, D. Sundholm, Phys. Chem. Chem. Phys. 13 (2011) 20500
[4] H. Fliegl, O. Lehtonen, D. Sundholm, V.R.I. Kaila, Phys. Chem. Chem. Phys. 13 (2011) 434
Dynamics
Florent Calvo
CNRS and University Joseph Fourier Grenoble, France
Modeling vibrational action spectroscopy: anharmonicities and quantum effects
Recent experimental progress in infrared spectroscopy has been driven by the development of free-electron lasers and the advent of infrared multiphoton dissociation (IRMPD) technique. With this method, a gas phase molecule is exposed to IR light and its dissociation is monitored by mass spectrometry, the measured signal being called an action (or depletion) spectrum. Interpretation of such spectra requires successful assignment by calculations of absorption spectra, usually carried out by density functional theory at the harmonic level. In this contribution we discuss and illustrate the fundamental differences between action and absorption spectra, and emphasize the crucial role played by anharmonicities and the way they are dynamically amplified by multiphoton processes. Our investigation relies on two separate but completary models that describe the interaction between the molecule and the laser field either classically through molecular dynamics at the atomistic level, or quantum mechanically through a Dunham expansion of the vibrational energy levels. For both models, specific spectral features such as additional redshifts or excessive broadenings are clearly identified.
Fabien Gatti
CTMM Institut Charles Gerhardt UMR-CNRS 5253 University of Montpellier, France.
and Hans-Dieter Meyer
Institute for Physical Chemistry, University of Heidelberg, Germany.
Full ab inito Molecular Quantum Dynamics with the Multi-Configuration Time-Dependent Hartree (MCTDH) method
We present the Multi-Configuration Time-Dependent Hartree (MCTDH) approach [1]. MCTDH is a general algorithm to solve the time-dependent Schr ̈odinger equation for multidimensional dynamical systems consisting of distinguishable particles. MCTDH can thus determine the quantal motion of the nuclei of a molecular system evolving on one or several coupled electronic potential energy surfaces. The possibilities offered by the Heidelberg MCTDH package [2] are also presented: calculation of photoabsorption spectra, cross sections, eigenstates and quantum resonances, dynamics around conical intersections, etc. Special emphasis is placed on the outlook for possible applications in chemistry in the context of coherent control with laser pulses.
[1] H.-D. Meyer, F. Gatti, and G. Worth, Multidimensional Quantum Dynamics: MCTDH Theory and Applications Wiley-VCH, 2009.
[2] See http://www.pci.uni-heidelberg.de/tc/usr/mctdh/
Michel A. Van Hove
Institute of Computational and Theoretical Studies & Department of Physics, Hong Kong Baptist University, Hong Kong SAR, China
Rotor molecules as machines
Molecular machines are gaining increasing interest, especially from a biological perspective. They promise to create and control mechanical motion at length scales down to the nanometer. Some molecular machines cause reciprocal motion, as in muscles and switches, while others cause rotational motion, as in flagellae: we focus here on rotor molecules.
Nature developed a variety of molecular machines to create and control motion. These natural machines tend to be complex and robust, due to the need to operate reliably for long times in variable biological environments.
In the last few decades, scientists have synthesized a wide range of new, relatively simpler molecular machines and learned to control and observe some of their important motions, mostly in solution. Increasingly, molecular motors have also been investigated at solid surfaces, allowing the use of surface science techniques for studying monolayers of well-oriented molecules. Nanoscience techniques have added further possibilities.
We shall discuss basic issues of the operation of molecular motors, including energy conversion steps, continuous energy supply, the role of thermal energy, intentional start and stop of motion, and unidirectionality of motion. Without intentional control of these aspects, motors create random motion and are largely useless.
This work was supported by grants from the Hong Kong Baptist University Strategic Development Fund, the Hong Kong RGC, the NBRPC and the NSFC, and by HKBU’s High Performance Cluster Computing Centre, which receives funding from the Hong Kong RGC, UGC and HKBU.
Benjamin Lasorne
Institut Charles Gerhardt, CNRS – Université Montpellier 2, 34095 Montpellier, France
and Marta Araújo,[1] Charlotte S. M. Allan,[2,3] David Mendive-Tapia,[2] Michael J. Bearpark,[2] Graham A. Worth,[3] Michael A. Robb[2]
[1] REQUIMTE, Fac. Ciencias do Porto, Dep. Quimica e Bioquimica, 4169-007 Porto, Portugal
[2] Department of Chemistry, Imperial College London, London SW7 2AZ, United Kingdom
[3] School of Chemistry, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom
Gaussian-Based Direct Quantum Dynamics Applied to Non-adiabatic Photochemistry
A serious bottleneck to quantum dynamics studies is the production of accurate potential energy surfaces represented on grids. Direct dynamics aims to free simulations from this preliminary step by calculating the potential energy and its derivatives on the fly. Non-adiabatic events such as internal conversion require a quantum treatment of the nuclear motion [1]. A variety of semiclassical methods have been developed over the years to simulate the wavepacket evolution in terms of swarms of trajectories. Unfortunately, they are expensive methods to converge.
Full quantum dynamical direct dynamics has more recently become viable using Gaussian wavepacket dynamics [2]. Implementations have been reported in the form of the ab initio multiple spawning (AIMS), the ab initio multiconfiguration Ehrenfest (AI-MCE) scheme, and the direct dynamics variational multiconfiguration Gaussian (DD-vMCG) wavepacket method presented here. The main advantages of the latter are that Gaussian basis functions are coupled directly and evolve according to a variational principle instead of following classical trajectories. As a result, the convergence properties are superior.
We present here three applications of the DD-vMCG method to the investigation of photochemical reactions: the photodissociation of formaldehyde [3], the photoisomerisation of a model cyanine [4], and the radiationless decay of fulvene [5].
Simulation results can be seen as a coupled set of quantum trajectories where a Mulliken-type population analysis helps to provide a straightforward interpretation of the wavepacket in much the same way as semiclassical trajectories are usually analysed.
[1] B. Lasorne, G. A. Worth, and Michael A. Robb, in: Molecular Quantum Dynamics – From Theory to Applications, F. Gatti (Ed.), Physical Chemistry in Action, (Springer, Heidelberg, 2014)
[2] G. A. Worth, M. A. Robb, and B. Lasorne, Mol. Phys. 106 (2008) 2077; B. Lasorne and G. A. Worth, in: Multidimensional Quantum Dynamics: MCTDH Theory and Applications, H.-D. Meyer, F. Gatti, and G. A. Worth (Eds.), (Wiley-VCH, Weinheim, 2009)
[3] M. Araújo, B. Lasorne, A. L. Magalhães, M. J. Bearpark, and M. A. Robb, J. Phys. Chem. A 114 (2010) 12016
[4] C. S. M. Allan, B. Lasorne, G. A. Worth, and M. A. Robb, J. Phys. Chem. A 114 (2010) 8713
[5] D. Mendive-Tapia, B. Lasorne, G. A. Worth, M. J. Bearpark, and M. A. Robb, Phys. Chem. Chem. Phys. 48 (2010) 15725; D. Mendive-Tapia, B. Lasorne, G. A. Worth, M. A. Robb, and M. J. Bearpark, J. Chem. Phys. 137 (2012) 22A548
Troy Van Voorhis
Massachusetts Institute of Technology
and Ben Kaduk, David McMahon, Shane Yost
Using Constraints to Build Active Spaces in DFT
One of the outstanding problems in describing low-lying excited states using density functional theory is the importance of static correlation. In wave function methods, this problem is often alleviated by using active space methods: CASSCF, RAS-CI, MCSCF and the like. These methods choose a handful of configurations or orbitals and treat the correlation in this space exactly using CI – thus obtaining a few low lying excited states in a manner that includes static correlation explicitly. Defining an active space in DFT is much harder as there are no orbitals, no configurations and one is implicitly limited to ground state calculations. I will outline some progress that has been made toward doing active space calculations in a DFT framework. Specifically, I will discuss: 1) How constrained DFT can be used to create diabatic states 2) The extent to which non-aufbau occupations of a Kohn-Sham reference are useful at representing locally excited configurations and 3) Methods by which these configurations can be coupled together to form an active space that describes states with multi-reference character. Time permitting, I will discuss some application of these ideas to the problem of singlet fission.
Computational biochemistry / Solvation
Aurelien de la Lande
Paris-Sud University, France
COMPUTATIONAL INVESTIGATION OF THE HYDROXYLATION MECHANISM OF NON-COUPLED COPPER OXYGENASES
In Nature peptidylglycine alpha-hydroxylating monooxygenase (PHM), Dopamine beta-monooxygenase and Tyramine beta-monooxygenase are known to achieve the remarkable dioxygen-dependent hydroxylation of aliphatic C-H bonds using two uncoupled copper sites.1 In spite of many investigations, either biochemical, chemical or computational, the details of the C-H bond oxygenation mechanism remain elusive; conflicting proposals have been advanced in the recent literature for the enzymatic catalytic cycle.2 I will present an investigation of the hydroxylation mechanism of PHM using ab initio Molecular Dynamics simulations based on hybrid Density Functional Theory-classical potentials (ie. QM/MM). This computational scheme permits the inclusion of the intrinsic dynamics of the active site into the modeling strategy. The major result of this study has been an extremely fast (picoseconds timescale) rebound after the initial H-abstraction step promoted by the cupric-superoxide adduct. The H-abstraction/Rebound sequence leads to the formation of an alkylhydroperoxide intermediate. A long range electron transfer from the remote copper site subsequently triggers its reduction to the hydroxy lated substrate.3 The characteristic time scale of the rebound step (ps) suggests that it is very probably the fastest reactive pathway over the previously advanced proposals. Moreover, our proposal finds favorable echoes with recent experimental results obtained on biomimetic complexes.5 Overall our study sheds new lights on how PHM achieves hydroxylation of C-H bonds with two uncoupled copper sites.
1: a) Osborne R.L.; Klinman, J. P. "Copper Oxygen Chemistry", pp 1-22; 2011, Ed. Karlin, K. D.; Itoh, S. Pub. J. Wiley & Sons. b) Solomon, E. I. et al. Chem. Rev. ASAP. DOI: 10.1021/cr400327t 2: see for example scheme 2 of N. R. McIntyre, E. W. Lowe Jr., D. J. Merkler, J. Am. Chem. Soc. 2009, 131, 10308 –10319. 3: Melía C., Ferrer S., Řezáč J., Parisel O., Reinaud O., Moliner V., de la Lande A. Chem. Eur. J. 2013, 19, 17328 – 17337. 4: de la Lande A., Parisel O., Moliner V. J. Am. Chem. Soc. 2007, 129,11700-11707. 5: a) Izzet, G., Zeitouny, J., Akdas-Killig, H., Frapart, Y., Ménage, S., Douziech, B., Jabin, Y., Le Mest, Y, Reinaud,O. J. Am. Chem. Soc. 2008, 130, 9514 –9523. b) Thiabaud, G. Guillemot, G., Schmitz-Afonso, I., Colasson, B. Reinaud, Angew. Chem. Int. Ed. 2009, 48, 7383 –7386. c) Tano, T., Sugimoto, H., Fujieda, N., Itoh, S. Eur. J. Inorg. Chem. 2012, 4099–4103.
Lars G.M. Pettersson
FYSIKUM, AlbaNova University Center, Stockholm University, S-106 91 Stockholm
Water - Structure and origin of its anomalous properties
I will discuss recent experimental and simulation data from x-ray absorption (XAS), emission (XES) and scattering (XRD and SAXS) of liquid water and the picture of fluctuations between high-density (HDL) and low-density (LDL) liquid this has led to [1,2]. Such a two-liquid scenario would explain many anomalous properties, e.g., density maximum, heat capacity and isothermal compressibility minima, but no direct connection has been found to simulations of ambient water. Applying the local structure index (LSI) of Shiratani and Sasai [J. Chem. Phys. 104, 7671 (1996)] to the inherent structure of TIP4P/2005 water we find a strict bimodality in terms of spatially separated HDL- and LDL-like environments in the simulations at all temperatures and pressures with distributions in agreement with the conclusions from XAS and XES, i.e. 75% HDL and 25% LDL-like at ambient conditions [3]. The SAXS signal is connected to density fluctuations in the liquid which for water decrease (as measured by the isothermal compressibility) down to 46 ˚C, but then increase as the liquid is further cooled. Going into “No man’s land” to do experimental measurements has recently become possible through the Linac Coherent Light Source (LCLS) x-ray free-electron laser at SLAC by exploiting evaporative cooling in vacuum of micrometer-sized water droplets from which a full diffraction pattern of individual droplets is obtained through the 100 fs fully coherent x-ray pulses delivered by the LCLS showing a continuous and accelerated development towards an LDL liquid down to at least 227 K [4].
[1] A. Nilsson and L. G. M. Pettersson, Perspective on the Structure of Liquid Water, Chem. Phys. 389, 1-34 (2011)
[2] A. Nilsson, C. Huang and L.G.M .Pettersson, Fluctuations in Ambient Water, J. Mol. Liq. 176, 2-16 (2012)
[3] K. T. Wikfeldt, A. Nilsson and L. G. M. Pettersson, Spatially Inhomogeneous Bimodal Inherent Structure in Simulated Liquid Water, Phys. Chem. Chem. Phys. 13, 19918-19924 (2011)
[4] J. A. Sellberg et al., Experimental observation of bulk liquid water structure in “no-man’s land”, Nature, in press.
Jean-Philip Piquemal
Affiliation
Title
Summary
G. Andres Cisneros
Department of Chemistry, Wayne State University, Detroit, MI 48202, USA
Development of accurate force fields for classical simulations
We have developed a novel force field, called the Gaussian Electrostatic Model (GEM), that employs explicit molecular charge densities. These densities are employed to calculate each term in the Morokuma-style decompositon of the quantum mechanical intermolecular interaction, i.e., Coulomb, exchange-repulsion, polarization, charge-transfer and dispersion. GEM enables the evaluation of intermolecular interactions for molecular systems with errors below chemical accuracy for each component, and provides a novel procedure to obtain distributed multipoles (GEM-DM). We will discuss the details of our method, advances in the implementation of a GEM variant for molecular dynamics simulations, recent applications of this variant for liquid water simulations, and applications of GEM-DM for the development of AMOEBA for ionic liquids simulations.
Julia Contreras-Garcia
Université Pierre et Marie Curie, Laboratoire de Chimie Théorique / CNRS
Revealing non covalent interactions in biosystems
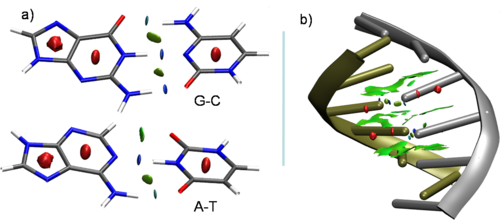
Molecular structure does not easily identify the intricate noncovalent interactions that govern many areas of biochemistry, including design of ligands, self-assembled materials, catalysts, drugs, and other molecular systems. We have developed an index, named NCI, to detect noncovalent interactions in real space. Although based on the electron density and its derivatives, it only requires knowledge of the atomic coordinates, so that it is highly efficient and applicable to large systems, such as proteins or DNA[1]. NCI provides a rapid and rich representation of van der Waals interactions (vdW), hydrogen bonds (HB), and steric clashes as low-gradient-low-density isosurfaces. Most importantly, the method, requiring only knowledge of the atomic coordinates, is efficient and applicable to large systems. Figs. 1a-b shows one such example for the different noncovalent interactions appearing in DNA. Colours coding is as follows: HBs are in blue, vdW interactions in green and steric clashes in red. The interactions between individual deoxyadenosine-deoxythymidine and deoxycytidine-deoxyguanosine are highlighted in Figure 1a. It can be seen that NCI is able to reveal the stronger nature of N-H-O vs C-H-O interactions. The large green regions are indicative of π-stacking between base-steps (Fig. 1b). Across numerous applications of NCI, a view of nonbonded interactions emerges as continuous surfaces rather than close contacts between atom pairs, providing insight into the multiple small contribution to macromolecular interaction. This tool hence offers exciting possibilities for aiding design of ligands, self-assembled materials, catalysts, and other molecular systems.
Fig.1 Non covalent interactions from NCI in AND: a) Interactions between G-C and A-T pairs b) Interactions within the B-form of double-strand of DNA
[1] E. R. Johnson, S. Keinan, P. Mori-Sanchez, J. Contreras-Garcia, A. J. Cohen, and W. Yang, “Revealing Noncovalent Interactions”, J. Am. Chem. Soc. 132 , 6498 (2010)
Guillaume Lamoureux
Department of Chemistry and Biochemistry and Centre for Research in Molecular Modeling, Concordia University, Montreal, Quebec H4B 1R6, Canada
Simulation of proton transfer and ion transport in proteins
Proton-coupled ion transport processes in membrane proteins present interesting challenges for molecular modeling. While the substrates being transported — protons (H+) and ions such as sodium (Na+) or ammonium (NH4+) — are small and interact with the protein in a highly specific (and selective) manner, the protein itself often undergoes large conformational changes during the transport cycle. How can one accurately simulate ion binding processes and proton transfer reactions in the dynamic environment of membrane proteins? I will be discussing some of the modeling approaches we have used to tackle the question, in the context of our work on the ammonium transporter AmtB and the sodium/proton antiporter NhaA.
Material science/ Catalysis
Alexis Markovits
Université Pierre et Marie Curie
Strong Metal Support Interaction: an electron count
Summary
Metal/Metal-oxide interface is an attractive topic in many aspects. It has been given considerable attention due to the various technological applications for electronic or photovoltaic devices, coating or sensor devices. The domain we are interested in is heterogeneous catalysis where the very special interaction between a support and a metal aggregate is known as Strong Metal Support Interaction (SMSI). The activation of the so-called SMSI catalyst is indispensable. Several explanations have been proposed since the discovery of SMSI as for instance metal dispersion allowing the formation of metal nanoparticules of various sizes and morphologies. Another explanation is the electronic effect. In this talk, we will mainly be focusing on this aspect. We take as an example CO dissociation that is important in many catalytic reactions. The first point is to evaluate and to rationalize the interaction strength between the metal M (M=K-Zn) and the reducible support TiO2. Then, CO dissociation on supported M is considered: is it assisted by hydrogenation? The influence of surface oxygen vacancy will be evaluated. The case of Iron supported on TiC support is developed: what is the influence of the support on CO dissociation? CO reactivity on bare and supported Fe clusters is compared.
Electron count and orbital analysis for understanding catalysts are supported by periodic DFT calculations. ↑ top of this page
Monica Calatayud
Univesité Pierre et Marie Curie
Exploring ceria-gallia mixed materials
Doping ceria is a strategy to obtain materials with outstanding properties. The presence of gallium atoms in the ceria lattice leads to an enhanced reducibility of the pure material. In this talk we will present a combined experimental and theoretical work aiming at characterizing the material and testing its properties in catalytic reactions.
[1] Unravelling the enhanced reactivity of bulk CeO2 doped with gallium: a periodic DFT study P. Quaino, L. Siffert, O. Syzgantseva, F. Tielens, C. Minot, M. Calatayud Chem. Phys. Lett. 519-520, (2012) 69
[2] Surface Reduction Mechanism of Cerium-Gallium Mixed Oxides With Enhanced Redox Properties J. Vecchietti, S. Collins, W. Xu, L. Barrio, D. Stacchiola, M. Calatayud, F. Tielens, F., Delgado, J., Bonivardi, A. J. Phys. Chem. C 117 (2013) 8822
[3] Understanding the role of oxygen vacancies in the water gas shift reaction on ceria-supported platinum catalysts J. Vecchietti, A. Bonivardi, W. Xu, D. Stacchiola, J. J. Delgado, M. Calatayud, S. Collins ACS Catalysis 4 (2014) 2088
Javier Fdez. Sanz
Universidad de Sevilla, Spain
Mechanism of the Water-Gas Shift Reaction: Insights from First Principles Calculations
The traditional approach to the optimization of metal/oxide catalysts has focused on the properties of the metal and the selection of the proper oxide for its dispersion. The importance of metal–oxide interfaces has long been recognized, but the molecular determination of their properties and role is only now emerging. In this talk we focus on the water gas shift reaction, WGSR, a chemical process that allows for obtaining clean molecular hydrogen: CO+H2O → CO2+H2. Bulk like phases or extended surfaces of coinage metals show low catalytic activity that improves when supported on a metal-oxide. Several reaction mechanisms have been proposed. In the redox mechanism, CO reacts with oxygen derived from the dissociation of H2O. In the associative process, the formation of a carboxyl intermediate must precede the production of H2 and CO2. The mechanism involves several steps that can take place at different sites of the catalyst: the metal, the support or the interface. Besides the dispersion effect, the role of the support is to increase the interaction with water and facilitate its dissociation. DF calculations show that supported CeOX nanoparticles are highly efficient in water splitting. Furthermore The M/CeOx /TiO2 (110) surfaces display outstanding activity for the WGS, in the sequence: Pt > Cu > Au. Such a high catalytic activity reflects the unique properties of the mixed-metal oxide at the nanometer level. STM and DF calculations show that Ce deposition on TiO2 (110) at low coverage gives rise to Ce2O3 dimers specifically aligned, indicating that the substrate imposes on the ceria NPs unusual coordination modes enhancing their chemical reactivity.
Frederik Tielens
Université Pierre et Marie Curie, Laboratoire Chimie de la Matière Condensée de Paris UPMC / CNRS, Collège de France
DFT Based Pharmacological and Medical Studies
The still continousely increase in calculation power enables the application of DFT to more and more complex systems. One of the most complex systems to be studied are encountered in life sciences. In this talk we will present our recent results on the chemistry and description of three examples: 1.Drug carriers, such as ibuprofene and alendronate encapsulated in amorphous silica particles, 2: The study of bone structure and bone generatation, and 3: Enraveling the composition, structure and reactivity of kidney stones, based on calcium oxalate. These studies are conducted in close collaboration with experimentalists.
References:
Colas, L. Bonhomme-Coury, C. Coelho, F. Tielens, F. Babonneau, C. Gervais, D. Bazin, D. Laurencin, M.E. Smith, J.V. Hanna, M. Daudon, and C. Bonhomme, Whewellite, CaC2O4.H2O: NMR and crystallography approach, CrystEngComm. 15, 8840 (2013).
N. Folliet, C. Roiland, S. Begu, A. Aubert, T. Mineva, A. Goursot, K. Selvraj, L. Duma, F. Tielens,F. Mauri, G. Laurent, C. Bonhomme, C. Gervais, F. Babonneau and T. Azaïs, Investigation of the Interface in Silica-Encapsulated Liposomes by Combining Solid State NMR and First Principles Calculations, J. Am. Chem. Soc. 133, 16815 (2011).
N. Folliet, C. Gervais, D. Costa, G. Laurent, F. Babonneau, L. Stievano, J.-F. Lambert, F. Tielens, A Molecular Picture of the Adsorption of Glycine in Mesoporous Silica through NMR Experiments Combined with DFT-D Calculations.J. Phys. Chem. C, 117, 4104, (2013).
Khuong P. Ong
Institute of High Performance Computing, A*STAR, Singapore
Hybrid organic – inorganic Perovskites
Hybrid organic- inorganic perovskites ABX3 has been attracting much concern recently due to its high photovoltaics conversion efficiency for solid state sensitised solar cell based on CH3NH3PbI3 hybrid perovskite. It can be considered as next generation of solid stae sensitised solar cells. In this talk, we will present first principles calculations on the effect of strain engineering on phase transitions of CH3NH3PbI3. The role of CH3NH3 moleculars on the crystal structure, electronic structure, and optical properties will be discussed.
Chemical concepts
Paul Ayers
Using molecular properties to define similarity measures and predict chemical properties
Jerzy Cioslowski
Institute of Physics, University of Szczecin, Poland
A tale of natural orbitals
Natural orbitals (NOs) and their occupancies constitute a convenient and conceptually appealing representation of the one-electron reduced density matrix from which all one-electron properties (including the majority of indices of interest to chemists) follow. However, their importance notwithstanding, properties of NOs are not fully understood. In particular, the existence of NOs with vanishing occupancies has been the subject of several recent studies with contradictory conclusions.
In this talk, after a brief introduction to properties of natural orbitals and their occupancies, we discuss results of our recent work uncovering the existence of solitonic NOs that resolves the apparent contradictions encountered in the previously published research. Both numerical calculations and analytical derivations are presented.
Eduard Matito
Aromaticity from Electron Delocalization Measures
In the last decades, the study of aromaticity has experienced an enormous progress. The new discoveries, which include species such as the metallabenzenes, heterometallabenzenes, metallabenzynes, metallabenzenoids, metallacyclopentadienes, metallacyclobutadienes, and all-metal and semimetal clusters, have joined the classical organic aromatic molecules such as benzene, benzenoid and nonbenzenoid polycyclic aromatic hydrocarbons, and heteroaromatic species to conform the current aromatic zoo. These new molecules, which are potentially useful for certain purposes as specific and very efficient catalysts, molecular electronic devices, molecular magnets, drugs, and other as yet unimagined applications, have brought a complete revolution in the field. At variance with the classical aromatic organic molecules that possess only π-electron delocalization, aromaticity in these new species is much more complex. These compounds have σ-, π-, δ-, and φ-electron delocalization. In addition, they can combine different types of aromaticity thus giving rise to double or triple aromaticity, the so-called multifold aromaticity. The new molecules can also have conflicting aromaticity, i.e., they can be aromatic in one component and antiaromatic in another.
Most of the old indicators are not valid to discuss the complex aromaticity of these novel compounds. The lack of reliable measures of aromaticity for these systems has triggered the development of more general and reliable indices that can be applied to both classical organic and inorganic aromatic compounds. In this lecture, we make a brief review of recently developed electronic aromaticity indices and their performance.
1.- Feixas F., Matito E., Poater J., Solà M.; Metalloaromaticity. WIREs Comput. Mol. Sci. 3, 105 (2013) http://dx.doi.org/doi:10.1002/wcms.1115
2.- Feixas F., Matito E., Duran M., Poater J., Solà M.; Aromaticity and electronic delocalization in all-metal clusters with single, double, and triple aromatic character. Theor. Chem. Acc. 128, 419 (2011) http://dx.doi.org/doi:10.1007/s00214-010-0805-8
3.- Feixas F., Jiménez-Halla J.O.C., Matito E., Poater J., Solà M.; A test to evaluate the performance of aromaticity descriptors in all-metal and semi-metal clusters. An appraisal of electronic and magnetic indicators of aromaticity. J. Chem. Theory Comput. 6, 1118 (2010) http://dx.doi.org/doi:10.1021/ct100034p
4.- Matito E., Solà M.; The Role of Electronic Delocalization in Transition Metal Complexes From the Electron Localization Function and the Quantum Theory of Atoms in Molecules. Chem. Coord. Rev. 253, 647-655 (2009) http://dx.doi.org/doi:10.1016/j.ccr.2008.10.003
5.- Feixas F., Matito E., Poater J., Solà M.; On the Performance of Some Aromaticity Indices: A Critical Assessment Using a Test Set. J. Comput. Chem. 29, 1543-1554 (2008) http://dx.doi.org/doi:10.1002/jcc.20914
6.- Matito E., Solà M., Salvador P., Duran M.; Electron Sharing Indexes at the Correlated Level. Application to Aromaticity Measures. Faraday Discuss. 135, 325-345 (2007) http://dx.doi.org/doi:10.1039/b605086g
7.- Some slides and notes can be found at: http://iqc.udg.edu/~eduard/master.html
8.- Software: http://ematito.webs.com/programs.htm
Patrick Bultinck
New insights on chemical reactivity descriptors from a matrix perspective
This lecture will focus on atoms in molecules, electrostatic potentials and chemical reactivity in the context of the breakdown of the conventional approaches due to the possibility of (near) degenerate states in molecules and crystals or inherent inadequacies in e.g., the Frontier Molecular Orbital picture. Can our trusted tools survive or do they need improving or must just be abandoned ? We show that in cases of (near) degeneracy, atomic charges become rather ill-defined as the electrostatic potential needs to be computed using degenerate perturbation theory (1). The effects are even more outspoken for the Fukui function (2), where in this case the Fukui matrix (3,4) is even more important.
1. Bultinck, P.; Cardenas, C.; Fuentealba, P.; Johnson, P.A.; Ayers, P.W. Atomic charges and the electrostatic potential are ill-defined in degenerate ground states. J. Chem. Theor. Comput., 2013, 9, 4779-4788.
2. Bultinck, P.; Cardenas, C.; Fuentealba, P.; Johnson, P.A.; Ayers, P.W. How to compute the Fukui matrix and function for (quasi-)degenerate states. J. Chem. Theory Comput., 2014, 10, 202–210.
3. Bultinck, P.; Clarisse, D.; Ayers, P.W., Carbo-Dorca, R. The Fukui matrix: a simple approach to the analysis of the Fukui function and its positive character. Phys. Chem. Chem. Phys., 2011, 13, 6110–6115.
4. Bultinck, P.; Van Neck, D.; Acke, G.; Ayers, P.W. Influence of electron correlation on the Fukui matrix and extension of frontier molecular orbital theory to correlated quantum chemical methods. Phys. Chem. Chem. Phys., 2012, 14, 2408 - 2416.
↑ top of this page
Angel M Pendas
Learning (and teaching) chemical bonding from the statistics of electron populations in spatial domains
Electrons are countable particles, so asking how many of them will lie in a given region of space makes perfect sense. We will show in this didactic presentation how examining the possible distributions of the N electrons of a molecule in atomic regions leads to interesting descriptions of chemical bonding. This statistical scheme may be applied equally to naïve models or to high level computational descriptions, making it suitable for teaching purposes in frehsman courses. ↑ top of this page
Kati Finzel
The ambiguity of the quantum stress tensor and its implication for bonding indicators
Since the electronic stress tensor is not uniquely defined, possible bonding indicators originating from the quantum stress tensor may inherit this ambiguity. The implication for stress tensor based quantities is studied based on a general formula of the stress tensor1 for three different types of indicators, namely the stress tensor eigenvalues at the bond critical point2, the spindle structure of the stress tensor2 and the scaled trace3, whereby the scaling function is proportional to the Thomas-Fermi term. It will be shown that all three indicators suffer from the ambiguity and to which extent the interpretation based on such indicators may change.
1) Anderson et al., J. Phys. Chem. A 114, 8884 (2010)
2) Finzel, Int. J. Quantum Chem. 114, 568 (2014)
3) Finzel, Kohout, Theor. Chem. Acc. 132, 1392 (2013)
Posters
Elmer Gastelo
UNIVERSIDAD NACIONAL DE INGENIERIA, PERÚ.
Photocatalytic activity of cobalt ferrite CoFe2O4-nanoparticles subjected to heat treatment
Cobalt ferrite nanoparticles synthetized by the sol-gel method [1] were obtained by iron salts FeCl2.4H2O and cobalt Co(NO3)2.6H2O as precursors, using cetyltrimethylammonium bromide (CTAB) as a surfactant. These were subjected to heat treatment for 3.4hours[2] at different temperatures 200°C, 275°C, 300°C, 350°C, 400°C, 550°C and 750°C. Subsequently these were characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) and photocatalytic activity on bacteria Escherichia coli strain ATCC 22922. The results XRD show diffractograms own cobalt ferrite for samples how obtained and calcined (200°C-550°C) but a phase change temperature on 750°C was observed, using the formula Debye-Scherrer has calculated the grain size of the particle obtained sizes 18.7± 3.3 nm for the sample how obtained and 22.8 ± 2.0 nm, 21.2±1.9nm, 19.2±1.4nm, 24.2±1.5nm, 23.8±2.0nm, 22.2±1.90nm, 68.8±13.1nm for the samples calcined at 200°C, 275°C, 300°C, 350°C, 400°C, 550°C and 750°C respectively. The morphology of the samples were studied by FE-SEM images, which show spherical nanoparticles with average size of 27.0±8.0nm, 29.4±14.5nm, 58.9±30.4nm, 47.1±26.0nm, 70.3±39.4nm, 35.9±24.7nm by samples calcined at 200°C, 275ºC, 300ºC, 350ºC, 400ºC and 550ºC respectively, for the sample calcined at 750°C morphology was shaped nanoflakes with a size of 190.7±61.1nm. Finally, the photocatalytic activity of the samples was studied to disinfection of bacteria Escherichia coli
[1] Juan M. de Oca, Ll. Chuquisengo, H. Alarcón, 2010. Síntesis y caracterización de nanopartículas de ferrita de cobalto obtenidas por el proceso sol-gel, Revista Sociedad Química Perú 76(4), 400-406.
[2] Sajjia, M., Benyounis, K.Y., Olabi, A.G., 2012. The simulation and optimization of heat treatment of cobalt ferrite nanoparticles prepared by the sol–gel technique. Powder Technology 222, 143–151.
[3] Casbeer, E., Sharma, V.K., Li, X.-Z., 2012. Synthesis and photocatalytic activity of ferrites under visible light: A review. Separation and Purification Technology 87, 1–14.
Alexey I. Baranov
Inorganic Chemistry II, Department of Chemistry and Food Chemistry, Dresden University of Technology, Max Planck Institute for Chemical Physics of Solids, Dresden, Germany.
Indicators for Quantitative Atomic Shell Structure Analysis from Fully Relativistic Calculations
One of the most fundamental concepts of chemistry is the concept of atomic shell structure. In the field of real space bonding analysis several bonding indicators have been proposed to reveal shell structure of chemical elements and thus visually represent key entities of chemical bonding like bonds or lone pairs. This type of analysis should be especially beneficial for relativistic 2c- or 4c-formalisms, eliminating the necessity of direct analysis of complicated multicomponent wavefunction.
This work presents an electron localizability indicator for spatially antisymmetrized electrons, which can be used to reveal an atomic shell structure at quantitative level in real space from the results of fully relativistic calculations. The indicator is universal and equally applicable for two-component and scalar-relativistic methods. Shell structures of heavy elements, calculated using this indicator from the results of fully relativistic, ZORA scalar relativistic and nonrelativistic numerical Kohn-Sham LDA calculations are reported and compared with each other. It was also applied on several molecular and crystalline systems to demonstrate the effect of spin-orbit coupling on the chemical bonding.
[1] Baranov, A. I. J. Comp. Chem. 2014, 35, 565.
Sunghwan Choi
Department of Chemistry, KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701, South Korea
Investigation of Lagrange-sinc Function as Basis Set for Density Functional Theory in terms of Chemical Accuracy
We developed a parallel self-consistent field program based on Kohn-Sham density functional theory using Lagrange-sinc functions as a basis set and examined its numerical accuracy for atoms and molecules through the comparison with the results of various Gaussian basis sets. The result of the Kohn-Sham inversion formula for the Lagrange-sinc functions manifests that the pseudopotential method is essential for cost-effective calculations. The Lagrange-sinc basis sets show rapid convergence of kinetic and correlation energies of benzene as the size of basis sets increase compared to the finite difference method, though both share the same uniform mesh. The mean absolute error in the atomization energies of the Lagrange-sinc basis for the G2-1 test set was comparable to that of AUG-cc-pV5Z among the Gaussian basis sets. Likewise, the two basis sets showed similar mean absolute errors in the ionization energies, the electron affinities, and the static polarizabilities of atoms in the G2-1 set. In particular, the Lagrange-sinc basis set showed high accuracy with rapid convergence in describing orbital changes by a strong external electric field, while Gaussian basis sets require a large set of diffuse functions with a slow convergence of an unoccupied orbital energy sensitive to the field. Overall the Lagrange-sinc basis shows reliable accuracy for electronic structure calculations of atoms and molecules.
Hung Tan Pham
Institute of Computational Science and Technology, Ho Chi Minh City, Vietnam
Theoretical models for structures of boron clusters
This work devotes to an understanding of the geometrical selection and electronic structure in some prototypical cases of boron clusters using simple theoretical models. The tubular forms such as B2n double ring, B27+, B42 triple ring can be predicted by model of a particle in hollow cylinder box. The model of circular box gives a good description to the planar and bowl shape B30, B36, B42 boron clusters. The transition from 2D (quasi-planar ) to 3D (double ring) can be understood by the charge effect.
Long Van Duong
Institute of Computational Science and Technology, Ho Chi Minh City, Vietnam
Title
Summary
[1] Popelier, P. L. A.; Brémond, É. A. G. Int.J.Quant.Chem. 2009, 109, 2542.
Phuong My Pham-Ho
Institute of Computational Science and Technology, Ho Chi Minh City, Vietnam
Title
Summary
[1] Popelier, P. L. A.; Brémond, É. A. G. Int.J.Quant.Chem. 2009, 109, 2542.
Tuyet Ngoc Thi Nguyen
Institute of Computational Science and Technology, Ho Chi Minh City, Vietnam
Title
Summary
[1] Popelier, P. L. A.; Brémond, É. A. G. Int.J.Quant.Chem. 2009, 109, 2542.
I. Urdaneta, A. Keller, O. Atabek, V. Mujica, M. Calatayud
LCT, UPMC (France), ISMO, UPSUD (France), IFUAP, BUAP (Mexico), DC&B, ASU (USA)
Understanding the SERS effect of dopamine adsorbed on titania nanoparticles
Recently it has been observed an important increase of the emitted Raman signal upon adsorption of dopamine on titania nanoparticles with respect to the bare molecule [1]. This phenomenon known as Surface Enhanced Raman Scattering (SERS) is quite common for metallic supports, but is by far less characterized for semiconducting materials. The mechanism explaining the enhancement of the Raman signal in the dopamine-TiO2 system has been postulated as a charge transfer one, involving an electron charge transfer from the molecule to the nanoparticle. The goal of the present work is to investigate the interface dopamine-TiO2 on an atomic level by means of Density Functional Theory (DFT) periodic calculations in order to elucidate the features connecting geometrical and electronic structures.
[1] Musumeci A. et al. J. Am. Chem. Soc, 2009, 131, 6040.
Nery Villegas-Escobar
LABORATORIO QUÍMICA TEÓRICA COMPUTACIONAL, FACULTAD DE QUÍMICA, PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE, .
Symmetry-Adapted Reaction Electronic Flux in Cycloaddition Reactions
The electronic activity along a reaction coordinate can be rationalized in terms of the Reaction Electronic Flux (REF). In this contribution its symmetry adapted extension is used to characterize the mechanism of two Diels-Alder reactions: butadiene plus ethylene to produce cyclohexene following a reaction path of Cs symmetry, and acetylene plus diacetylene to form o-benzyne in a C2v reaction path. In the case of the former reaction, the electronic activity is captured in terms of the symmetry adapted REF (SA-REF) according to irreducible representations A' and A". The symmetric representation characterizes the electronic activity due to pi-electronic reordering while the antisymmetric representation emcompasses the formation of the new sigma bonds. The more complex o-benzyne formation displays four SA-REFs associated to each of the irreducible representation of the C2v group. Results show that SA-REFs appears to be very useful for identifying electronic activity that displays an specific symmetry character. In the first reaction the pi-electronic activity drive the formation of cyclohexene whereas in the second reaction the in plane electronic activity drives the formation of o-benzyne.
[1] Villegas-Escobar, N., Vogt-Geisse, S., Gutiérrez-Oliva, S., & Toro-Labbé, A. (2016). Symmetry-adapted reaction electronic flux in cycloaddition reactions. Theoretical Chemistry Accounts, 135(8), 1-8.