Différences entre les versions de « VBTutorial2 »
| Ligne 428 : | Ligne 428 : | ||
== Exercise 3 : Resonance energy of Benzene == | == Exercise 3 : Resonance energy of Benzene == | ||
| − | |||
| − | |||
# Covalent state of benzene : | # Covalent state of benzene : | ||
## Propose a complete basis of non-redondant covalent VB structures for benzene. (hint there should be 5 structures). | ## Propose a complete basis of non-redondant covalent VB structures for benzene. (hint there should be 5 structures). | ||
| Ligne 436 : | Ligne 434 : | ||
# Full description of benzene : | # Full description of benzene : | ||
## Compute a VBSCF wave function including the complete set of VB structures, using the "str=full" keyword. How many structures are available overall? | ## Compute a VBSCF wave function including the complete set of VB structures, using the "str=full" keyword. How many structures are available overall? | ||
| − | ## In principle in order to calcuate properly the VRE one has to compute the energy difference between the overall adiabatic state of benzene and a corresponding Kékulé diabatic state. The latter Kékulé state requires an overall of 27 structures for its proper description, which have to be hand-written(!) One solution is the use of semi-localized orbitals offered by the "Bond Distorted Orbitals" option. Calculate the energy of one Kékulé state using the BDO (see BDO | + | ## In principle in order to calcuate properly the VRE one has to compute the energy difference between the overall adiabatic state of benzene and a corresponding Kékulé diabatic state. The latter Kékulé state requires an overall of 27 structures for its proper description, which have to be hand-written(!) One solution is the use of semi-localized orbitals offered by the "Bond Distorted Orbitals" option. Calculate the energy of one Kékulé state using the BDO (see BDO procedure below). Compare the energy of the covalent Kékulé structure calculated with HAO (hybrid atomic orbitals) and BDO. What is the reason for the difference? |
## Estimate the corresponding VRE, has it improved? | ## Estimate the corresponding VRE, has it improved? | ||
{| class="collapsible collapsed wikitable" | {| class="collapsible collapsed wikitable" | ||
|- | |- | ||
| − | !''BDO | + | !''BDO procedure'' |
|- | |- | ||
| | | | ||
| Ligne 454 : | Ligne 452 : | ||
|} | |} | ||
| + | {| class="collapsible collapsed wikitable" | ||
| + | |- | ||
| + | !'''Answer''' | ||
| + | |- | ||
| + | | | ||
| + | ====''Covalent state of benzene''==== | ||
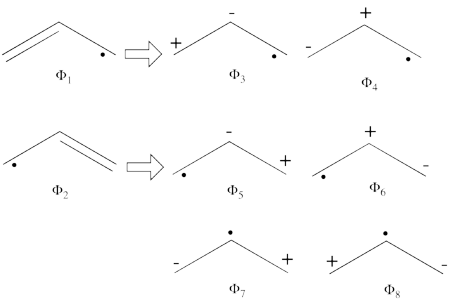
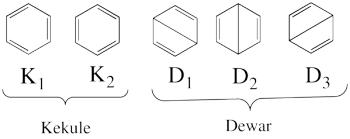
| + | * The following are the five covalent structures of the benzene: <br/>[[File:Benzene_5CovStruct.png|350px]]<br> | ||
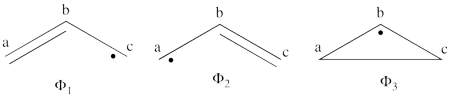
| + | These structures consist of 2 Kekule (<math> K_1 </math> and <math> K_2 </math>) and 3 Dewar (<math> D_1, </math> <math> D_2 </math> and <math> D_3 </math>) structures. | ||
| + | <br> | ||
| + | <br> | ||
| + | * VB energies of benzene's covalent state are summarized in the following Table: | ||
| + | {| border="1" | ||
| + | |+ | ||
| + | ! scope="col" | Structures & Method | ||
| + | ! scope="col" | Total Energy (au) | ||
| + | |- | ||
| + | ! scope="row" | 5 covalent (VBSCF) | ||
| + | | -230.54451931 | ||
| + | |- | ||
| + | ! scope="row" | 1 covalent (VBSCF) | ||
| + | | -230.49584123 | ||
| + | |- | ||
| + | ! scope="row" | 5 covalent(BOVB) | ||
| + | | -230.545495 | ||
| + | |} | ||
| − | + | The vertical resonance energy can be obtained by the difference between the energy of the state which includes all 5 covalent structures and only 1 Kekule structure. Thus, this VRE is calculated to be '''VRE''' = 30.55 kcal/mol (VBSCF) and 31.16 kcal/mol (BOVB). These values are too low when compared to the experimental value which is 65 kcal/mol (estimated by use of thermochemistry and empirical force constant. Chemical Review, '''101''', 1501-1539 (2001)) | |
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | ====''Full description of benzene''==== | ||
| + | # The complete number of the ionic structures is too large to be written by hand. So using the keyword "str=full" produces all covalent and ionic structures. For benzene this involves overall of 175 structures. | ||
| + | # In order to calculate the Kékulé diabatic state one has to calculate the Kékulé covalent structure along with all the related ionic structures (overall of 27 structures in this case). The keyword "str=full" cannot be used in this case, thus, in order to avoid tedious work we utilize the bond distorted orbitals '''bdo''' while calculating only the covalent structure. <br> The following Table summarizes the energies for the diabatic Kékulé state as well as the adiabatic state of benzene: | ||
| + | <br> | ||
| + | |||
| + | {| border="1" | ||
| + | |+ diabatic and adiabatic energies of benzene | ||
| + | ! scope="col" | Structures & Method | ||
| + | ! scope="col" | Total Energy (au) | ||
| + | |- | ||
| + | ! scope="row" | 1 Kekule ('''orbtyp=hao''') (VBSCF) | ||
| + | | -230.49584123 | ||
| + | |- | ||
| + | ! scope="row" | 1 Kekule ('''orbtyp=bdo''') (VBSCF) | ||
| + | | -230.58675751 | ||
| + | |- | ||
| + | ! scope="row" | 175 structures ('''str=full''') (VBSCF) | ||
| + | | -230.69492368 | ||
| + | |} | ||
| + | <br> | ||
| + | * The covalent Kékulé structure calculated with HAO (hybrid atomic orbitals) is 57 kcal/mol higher in energy than that calculated with the bond distorten orbitals (BDO). The reason is that the BDO calculation imcludes implicitely all the related ionic structures, which are important for the description of the state. | ||
| + | * The resonance energy '''RE''' = 67.9 kcal/mol (VBSCF) which is in good agreement with the experimental value (65 kcal/mol) | ||
| + | |} | ||
== Exercise 4 : The allyl cation == | == Exercise 4 : The allyl cation == | ||
Version du 12 juillet 2012 à 10:03
VB applications on PI systems
In all the following exercises, <math>\pi</math> the system will be taken as active, and the <math>\sigma</math> system as inactive. In all VB calculations, the <math>\sigma</math> orbitals shall be described by MOs delocalized onto the whole molecule.
| Main exercises | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Exercise 1 : The allyl radical
Exercise 2 : Radical character of ozone
|
| Optional Exercises - Homework | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Exercise 3 : Resonance energy of Benzene
Exercise 4 : The allyl cation
Computer Exercise
|