Différences entre les versions de « VBTutorial2 »
Aller à la navigation
Aller à la recherche
| Ligne 36 : | Ligne 36 : | ||
{| class="collapsible collapsed wikitable" | {| class="collapsible collapsed wikitable" | ||
|- | |- | ||
| − | !'''Answer''' | + | !'''Answer''' |
|- | |- | ||
| | | | ||
| Ligne 52 : | Ligne 52 : | ||
</math><br> | </math><br> | ||
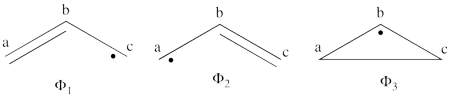
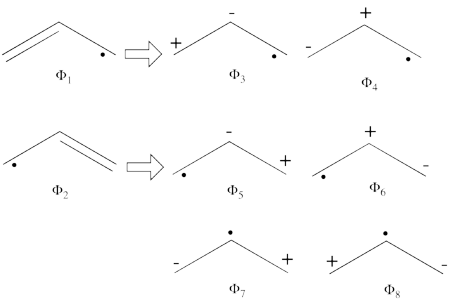
<br>Since <math> \Phi_{\textrm{3}} </math> is the positive combination of <math> \Phi_{\textrm{1}} </math> and <math> \Phi_{\textrm{2}} </math> the two first structures form a complete basis of non-redundant structures. | <br>Since <math> \Phi_{\textrm{3}} </math> is the positive combination of <math> \Phi_{\textrm{1}} </math> and <math> \Phi_{\textrm{2}} </math> the two first structures form a complete basis of non-redundant structures. | ||
| − | |||
<br> | <br> | ||
| Ligne 90 : | Ligne 89 : | ||
====3. ''Covalent and ionic structures of the allyl radical:'' ==== | ====3. ''Covalent and ionic structures of the allyl radical:'' ==== | ||
| − | #From each covalent structure we can get two ionic structures. Additional two ionic structures are obtained from the redundant structure. Therefore overall there are 6 different ionic structures <br>[[File:Allyl_Ionic-N1.png|450px]] <br> In the two ionic structures (<math> \Phi_{\textrm{7}} </math>,<math> \Phi_{\textrm{8}} </math>) that are based on the redundant structure the charges are farther from each | + | #From each covalent structure we can get two ionic structures. Additional two ionic structures are obtained from the redundant structure. Therefore overall there are 6 different ionic structures <br>[[File:Allyl_Ionic-N1.png|450px]] <br> In the two ionic structures (<math> \Phi_{\textrm{7}} </math>,<math> \Phi_{\textrm{8}} </math>) that are based on the redundant structure the charges are farther from each other compared with the other ionic structures and are therefore, expected to be higher in energy. These two structures will therefore not be included in our chosen sub-set of structures. |
|} | |} | ||
| − | |||
|} | |} | ||
Version du 12 juillet 2012 à 09:33
VB applications on PI systems
In all the following exercises, <math>\pi</math> the system will be taken as active, and the <math>\sigma</math> system as inactive. In all VB calculations, the <math>\sigma</math> orbitals shall be described by MOs delocalized onto the whole molecule.
| Main exercises | ||||
|---|---|---|---|---|
Exercise 1 : The allyl radical
Exercise 2 : Radical character of ozoneComputer Exercises
\psi_{H\ddot{u}ckel} = 25%\Phi_1 +
25%\Phi_2 + 12.5%\Phi_3 +
25%\Phi_4 + 6.25%\Phi_5 +
6.25%\Phi_6
</math> Paper Exercises - Optional/Homework
|
| Optional Exercises - Homework | ||
|---|---|---|
Exercise 3 : Resonance energy of BenzeneComputer Exercise
Exercise 4 : The allyl cation
Computer Exercise
|