Différences entre les versions de « VBTutorial2 »
| Ligne 111 : | Ligne 111 : | ||
## Compute this wave function at VBSCF then BOVB levels. Deduce what is the resonance energy of the allyl radical at the BOVB level. The resonance energy is calculated as the difference between the adiabatic state and the ''Lewis'' (diabatic) state of the allyl radical. | ## Compute this wave function at VBSCF then BOVB levels. Deduce what is the resonance energy of the allyl radical at the BOVB level. The resonance energy is calculated as the difference between the adiabatic state and the ''Lewis'' (diabatic) state of the allyl radical. | ||
| − | + | {| class="collapsible collapsed wikitable" | |
| + | |- | ||
| + | !'''Answer''' | ||
| + | |- | ||
| + | | | ||
| + | ====''1. Computation of covalent state of allyl radical''==== | ||
| + | # The weights of the two structures, <math> \Phi_1 </math> and <math> \Phi_{\textrm{2}} </math> is identical and equals 0.5 as expected. | ||
| + | # The wavefunction in determinant description is: <br> <math> | ||
| + | |||
| + | \psi_{GS}= -0.37\vert bc\bar{a}\vert - 0.78\vert ac\bar{b}\vert + 0.37\vert ba\bar{c}\vert | ||
| + | |||
| + | </math> <br> Rearrangement of the determinants results with the following wavefunction: <br> <math> | ||
| + | |||
| + | \psi_{GS}= 0.78\vert a\bar{b}c\vert - 0.37\vert \bar{a}bc\vert - 0.37\vert ab\bar{c}\vert | ||
| + | |||
| + | </math> <br> which is identical to the wavefunction derived in Paper Ex. 2.1. <br> Similarly, the spin pupolation obtained in the calculation is 0.667, -0.333, 0.667 for carbons a, b and c respectively in agreemnet with the results obtained in Paper Ex. 2.1. | ||
| + | # The wavefunction in the first excited state is: <br> <math> | ||
| + | |||
| + | \psi_{EX}= \Phi_1 + \Phi_2 = 0.71\vert bc\bar{a}\vert + 0.71\vert ba\bar{c}\vert | ||
| + | |||
| + | </math> <br> Again, rearrangement of the determinants results with the following wavefunction: <br> <math> | ||
| + | |||
| + | \psi_{GS}= 0.71\vert \bar{a}bc\vert - 0.71\vert ab\bar{c}\vert | ||
| + | |||
| + | </math> <br> and the corresponding spin population is: 1.0 on the central carbon as is expected for the long bond structure predicted in paper Ex. 2.3. | ||
| + | |||
| + | ====2. ''Computation of the allyl radical energies and weights''==== | ||
| + | * The total energy of the VB wave function with selected set of structures<br/> | ||
| + | |||
| + | {| border="1" | ||
| + | |+ VB energies of the allyl radical | ||
| + | ! scope="col" | Structures & Method | ||
| + | ! scope="col" | Total Energy (au) | ||
| + | |- | ||
| + | ! scope="row" | 2 cov + 4 ionic (VBSCF) | ||
| + | | -116.37308766 | ||
| + | |- | ||
| + | ! scope="row" | Str=full (VBSCF) | ||
| + | | -116.37668662 | ||
| + | |- | ||
| + | ! scope="row" | 2 cov + 4 ionic (BOVB) | ||
| + | | -116.39521431 | ||
| + | |} | ||
| + | The energy difference is -2.25 kcal/mol within the VBSCF. Comparison of VBSCF with BOVB both only using the selected subset, one gets a difference of -13.88 kcal/mol | ||
| + | <br> | ||
| + | <br> | ||
| + | * Comparison of the weights of the VB wavefunction: | ||
| + | |||
| + | {| border="1" | ||
| + | |+ Weights of the VB structures of the allyl radical | ||
| + | ! scope="col" | | ||
| + | ! scope="col" | Str=full (VBSCF) | ||
| + | ! scope="col" | 2 cov + 4 ion (VBSCF) | ||
| + | ! scope="col" | 2 cov + 4 ion (BOVB) | ||
| + | |- | ||
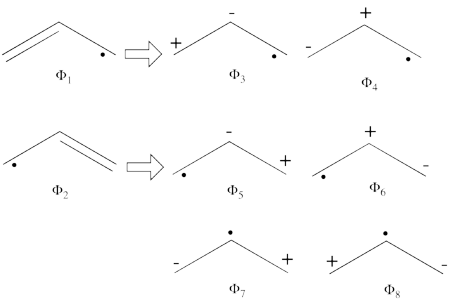
| + | ! scope="row" |<math>\phi_1</math> 1:10 11 12 13 | ||
| + | | 0.36392 || 0.37056 || 0.32495 | ||
| + | |- | ||
| + | ! scope="row" | <math> \phi_2 </math> 1:10 12 13 11 | ||
| + | | 0.36392 || 0.37056 || 0.32495 | ||
| + | |- | ||
| + | ! scope="row" | <math> \phi_3 </math> 1:10 12 12 13 | ||
| + | | 0.05844 || 0.06257 || 0.08716 | ||
| + | |- | ||
| + | ! scope="row" | <math>\phi_4 </math> 1:10 11 11 13 | ||
| + | | 0.06454 || 0.06687 || 0.08789 | ||
| + | |- | ||
| + | ! scope="row" | <math> \phi_5 </math> 1:10 12 12 11 | ||
| + | | 0.05844 || 0.06257 || 0.08716 | ||
| + | |- | ||
| + | ! scope="row" | <math> \phi_6 </math> 1:10 13 13 11 | ||
| + | | 0.06454 || 0.06687 || 0.08789 | ||
| + | |- | ||
| + | ! scope="row" | <math> \phi_7 </math> 1:10 11 11 12 | ||
| + | | 0.01310 | ||
| + | |- | ||
| + | ! scope="row" | <math> \phi_8 </math> 1:10 13 13 12 | ||
| + | | 0.01310 | ||
| + | |} | ||
| + | When comparing 2 cov + 4 ion with Str=full we see that the weights of the last two structures in the second calculation (the more comprehensive calculation) are indeed very small. | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | ==== ''3. Computation of resonance energies for the Allyl radical'' ==== | ||
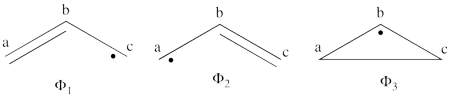
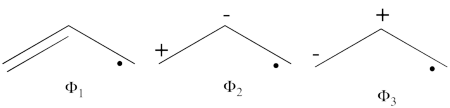
| + | #The following are the three structures of allyl radical which describe one of its Lewis structures (the structure which involves a bond between atoms a and b and an unpaired electron on atom c):<br>[[File:Allyl_Lewis.png|450px]] <br> <br> | ||
| + | #The total energy of the VB wave function of this Lewis structure is summarized in the following table: <br> <br> | ||
| + | {| border="1" | ||
| + | |+ Total energies and weights of one of the Lewis structures of the allyl radical | ||
| + | ! scope="col" | | ||
| + | ! scope="col" | VBSCF | ||
| + | ! scope="col" | BOVB | ||
| + | |- | ||
| + | ! scope="row" | Total energy (au) | ||
| + | | -116.34255135 || -116.359365 | ||
| + | |- | ||
| + | ! scope="row" | weight of <math> \Phi_1 </math> | ||
| + | | 0.80496 || 0.73157 | ||
| + | |- | ||
| + | ! scope="row" | weight of <math> \Phi_2 </math> | ||
| + | | 0.09494 || 0.13011 | ||
| + | |- | ||
| + | ! scope="row" | weight of <math>\Phi_3 </math> | ||
| + | | 0.10010 || 0.13832 | ||
| + | |} <br> At the BOVB level, the resonance energy (RE) of the allyl radical which is the difference between the total energy of the allyl radical with full set of the structures and the total energy of the Lewis structure only is RE = 22.5 kcal/mol. | ||
| + | |} | ||
| + | |||
|} | |} | ||
Version du 12 juillet 2012 à 09:36
VB applications on PI systems
In all the following exercises, <math>\pi</math> the system will be taken as active, and the <math>\sigma</math> system as inactive. In all VB calculations, the <math>\sigma</math> orbitals shall be described by MOs delocalized onto the whole molecule.
| Main exercises | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Exercise 1 : The allyl radical
Exercise 2 : Radical character of ozoneComputer Exercises
\psi_{H\ddot{u}ckel} = 25%\Phi_1 +
25%\Phi_2 + 12.5%\Phi_3 +
25%\Phi_4 + 6.25%\Phi_5 +
6.25%\Phi_6
</math> Paper Exercises - Optional/Homework
|
| Optional Exercises - Homework | ||
|---|---|---|
Exercise 3 : Resonance energy of BenzeneComputer Exercise
Exercise 4 : The allyl cation
Computer Exercise
|