Abstracts of the CTTC 2016
<<< CTTC 2016 workshop main page
Slides available after the workshop
HOW TO UPLOAD YOUR ABSTRACT
In order to upload your abstract, you will need a valid login account.
Please contact us email and we will email you your login details.
Please be aware that the whole system has been reinitialized, so old accounts are not valid anymore.
CONTRIBUTORS: please add below, in your own section, your title talk and abstract :
- first : log in; (contact the organization for log in details or use the ones we provided in 2014 if you attended Vietnam)
- click on your name in the "Contents" box below, this will lead you to your own section;
- your section starts with your name as the title line, click on [edit] (far right).
- >>> How to insert a picture in your abstract
- SPEAKERS: A template has been created with you name for you to fill in. Please upload your abstracts before 31st April 2016.
- POSTERS: If you wish to contributethe with a poster, feel free to follow the prescribed template in the Poster section . Please upload your abstracts before 30th July 2016.
All students are strongly encouraged to present a poster at the conference.
Speakers
Mercedes Alonso
Vrije Univ., Belgium
Expanded Porphyrins: from Understanding to Rational Design
In this talk, the use of computational chemistry to reveal the critical factors controlling the structure and properties of expanded porphyrins in different environments is presented. Through extensive density functional theory calculations, we recently demonstrated that the molecular topology is highly influenced by the number of π-electrons and the size of the macrocycle.[1] Aromaticity emerged as the key concept determining the electronic, transport and nonlinear optical properties of expanded porphyrins and accordingly, we proposed different methods to quantify the Hückel and the Möbius aromaticity. By using these descriptors, the structure-property relationships between the molecular topology, aromaticity and nonlinear optical properties was established and the optimum conditions for viable Möbius systems and optical switches were determined.[4]
Finally, I will show how quantum-chemical methods can be used for understanding and predicting the metalation effect in expanded porphyrins. Using energy decomposition analysis, we have recently found that the molecular topology of d8 metal complexes of hexaphyrins depends on the sensitive interplay between the intrinsic ligand strain and the metal-ligand interaction strain. As such, aromaticity of the ligand and effective charge of the metal are revealed as key factors determining the binding mode and the preference for Möbius or Hückel structures. These findings offer a new perspective to rationalize the experimental observations and we proposed several guidelines for designing novel complexes of hexaphyrins.[5]
[1] Alonso, M.; Geerlings, P.; De Proft, F. Chem. Eur. J. 2012, 18, 10916; Chem. Eur. J. 2013, 19, 1617; J. Org. Chem. 78, 4419 (2013). [2] a) Alonso, M.; Geerlings, P.; De Proft, F. Phys. Chem. Chem. Phys. 2014, 16, 14396; b) Woller, T.; Contreras-García; J.; Geerlings, P.; De Proft, F.; Alonso, M. Phys. Chem. Chem. Phys. 2016, 18, 11885.[3] Alonso, M.; Balazs, P.; Geerlings, P.; De Proft, F. Chem. Eur. J. 2015, 21, 17631.
Ramiro Arratia-Perez
Universidad Andrés Bello, Chile
Molecular Sensors for Diagnostic
We have synthesized Re organometallic complexes and further characterized by X-ray diffraction, NMR, IR, UV, electrochemistry and relativistic DFT calculations. We explored their intracellular localization by taking advantage of its revealed luminescence. We have developed several biological applications in the fields of precision agriculture and public health issues. In particular, we have designed molecular sensors for the detection of Botrytis cinerea, and we have synthesized a molecular compound that inhibits the growth of this fungus that attack grapes, pepper, tomatoes, cannabis, etc. This finding have the potential for establishing a protocol in precision agriculture. We also assessed the antimicrobial and bactericide activities of some of our organometallic complexes in bacteria (Salmonella enterica) and yeast (Cryptococcus spp., Candida albicans and Candida tropicalis)that affect human beings. We observed that our complexes exerts antifungal effects against Crytococcus spp. and has proven to be suitable markers for bacteria and yeast sensing. We determined their minimal inhibitory concentration (MIC).
[1] A. Carreno, R. Arratia-Perez et al. New J. Chem. 2015, 39, 7822-7831.
[2] A. Carreno, R. Arratia-Perez et al. New J. Chem. 2016, 40, 2362-2375.
[3] A. Carreno, R. Arratia-Perez et al. New J. Chem. 2015, 39, 5725-5734.
Acknowledgement. We thank Millennium Nucleus 120001 for financial support.
↑ top of this page
Paul Ayers
McMaster Univ., Canada
Strong Electron Correlation
Since the electronic Schrödinger equation is too complicated to be soluble for most interesting chemical systems, the task of the quantum chemist is to develop practical approximations that provide accurate models for the behavior of electrons in molecules. The difficulty of the underlying problem implies that these models are necessarily limited to certain special cases. For example, it is relatively easy to describe cases where the electrons in a molecule move nearly independently, so that the motion of one electron does not affect others very much. When this is not true, many of our conceptual precepts lose their utility (e.g., the notion of an electron configuration, and even the mere concept of orbitals) and many popular computational quantum chemistry methods become unreliable. In this lecture, I will discuss quantum chemical models for strongly correlated molecules, focusing on alternatives to orbital-based models.
Roi Baer
Fritz Haber Center for Molecular Dynamics, Institute of Chemistry, The Hebrew University of Jerusalem, Israel
Charged and neutral excitations in molecular and nanoscale systems
We present theoretical and computational approaches for studying quasiparticle and optical excitation in large systems. We start with first-principles tuning of range-separated hybrid (RSH) functionals, a technique based on density functional theory and then we introduce a stochastic approach to G0W0 and the Bethe Salpeter Equation, based on many-body perturbation theory. We show that RSH tuning allows for practical and effective correction of many ailments of DFT-based calculations but as system size grow its efficacy diminishes unless quasiparticles localize. Recently we found that in finite 1D polymers, such as polythiophene or trans polyacetylene quasiparticles localize with length scales of dozens of nanometers due to a purely electronic effect. Results from G0W0, applied to these large polymers strands seem to support this conclusion.
See Also:
- V. Vlček, H. R. Eisenberg, G. Steinle-Neumann E. Rabani, D. Neuhauser and R. Baer, Spontaneous charge-carrier localization in extended one-dimensional systems, Phys. Rev. Lett. 116, 186401 (2016).
- E. Rabani, R. Baer, and D. Neuhauser, Time-dependent Stochastic Bethe-Salpeter Approach, Phys. Rev. B 91, 235302 (2015).
- D. Neuhauser, Y. Gao, C. Arntsen, C. Karshenas, E. Rabani and R. Baer, Breaking the theoretical scaling limit for predicting quasi-particle energies: The stochastic GW approach, Phys. Rev. Lett. 113, 076402 (2014).
- R. Baer, D. Neuhauser, E. Rabani Self-averaging stochastic Kohn-Sham density functional theory, Phys. Rev. Lett. 111, 106402 (2013).
- L. Kronik, T. Stein, S. Refaely-Abramson and R.Baer Excitation gaps of finite-sized systems from Optimally-Tuned Range-Separated Hybrid Functionals, JCTC, 8, 1515 (2012).
- R. Baer, E. Livshits and U. Salzner, Tuned Range-separated hybrids in density functional theory, Ann. Rev. of Phys. Chem., 61, 85 (2010).
Evert Jan Baerends
VU University Amsterdam
Orbitals and orbital energies in DFT and TDDFT
Orbital energies in the common LDA and GGA calculations are very wrong: they are typically 5 eV (more than 100 kcal/mol) higher than the exact Kohn-Sham orbital energies, an error that would be completely unacceptable in total energies. This error has not prevented quite decent results for excitation energy calculations from the early days of applications of DFT (Xa) onwards. We will first analyze where this error comes from - it is not due to wrong asymptotic behavior of LDA/GGA potentials, or to a “self-interaction error” but it is caused by erroneous density dependence of the standard functionals, hence a wrong derivative (= potential). We will propose improved potentials [1], and will argue that orbitals and orbital energies obtained with good Kohn-Sham potentials have many virtues: 1) the orbital energies of the occupied orbitals are close to ionization potentials (an order of magnitude better agreement than the Koopmans’ type agreement in Hartee-Fock); 2) virtual orbital energies are realistic: occupied -unoccupied orbital energy differencies are very close to excitation energies. There is no “gap problem” in DFT! [2] 3) the KS virtual orbitals are typically bound and have good (valence type) shapes (not unphysically diffuse like the Hartree-Fock virtuals); excitations can be described in most cases as simple single orbital-to-orbital transitions.
It is possible to define suitable orbitals for the description of excitations in different electronic structure theories than DFT, such as TDHF (RPA) or TD Density Matrix FT. They turn out to be remarkably similar to the orbitals of the Kohn-Sham molecular orbital model [3].
[1] O. Gritsenko, E. J. Baerends, J. Chem. Phys. 144 (2016) 204114.
[2] E. J. Baerends, O. Gritsenko, R. van Meer, PCCP 15 (2013) 16408.
[3] R. van Meer, O. V. Gritsenko, E. J. Baerends, Chem. Phys. Lett. 639 (2015) 315.
Kati Finzel
Linköping Univ., Sweden
Recent advances in orbital-free density functional theory
Orbital-free density functional theory (OF-DFT) promises a reliable physical description at relatively low computational cost [1,2]. However, progress was hampered due to an insufficient knowledge how to model the Pauli exclusion principle within an orbital-free approach, consequently leading to structureless bosonic-like electron densities. Recently, the mathematic connection between the atomic shell structure in real space and the Pauli exclusion principle has been established for any set of (1s,2s)-orbitals [3]. Reversely, imposing local conditions on the Pauli potential [4] for a proper representation of the atomic shell structure assures properly structured electron densities from variational orbital-free calculations [4,5]. The method is applicable to all atoms in the Periodic Table [6] and extendable to bound Coulomb systems [7].
[1] P. Hohenberg, W. Kohn, Phys. Rev. B 1964, 136, 864. [2] M Levy, J. P. Perdew, V. Sahni, Phys. Rev. A 1984, 30, 2745. [3] K. Finzel, Theor. Chem. Acc. 2016, 135, 148. [4] K. Finzel, J. Chem. Phys. 2016, 144, 034108. [5] K. Finzel, Int. J. Quantum Chem. 2015, 115, 1629. [6] K. Finzel, Theor. Chem. Acc. 2016, 135, 87. [7] K. Finzel, Int. J. Quantum Chem. 2016, doi: 10.1002/qua.25169.
Marc Garcia-Borràs
Department of Chemistry and Biochemistry, University of California, Los Angeles
Computational tools for the evaluation of laboratory-engineered biocatalysts
After billions of years of evolution, Nature has evolved Enzymes conferring them unique capabilities fundamental for life. Enzymes (i.e. biocatalysts) are highly efficient, specific and selective. Indeed, they are the most proficient catalysts known. Biocatalysts can operate under physiological mild conditions, are biodegradable and non-toxic, offering exquisite control of biochemical transformations. All these characteristics make biocatalysts the best alternative to synthetic reagents for chemical manufacturing. Nevertheless, the existing enzymes in nature are specifically evolved to optimally operate on natural substrates catalyzing chemical transformations that may not be of industrial interest. Thus, because of this limit scope of substrates and synthetically useful chemical transformations, enzymes need to be modified to accomplish that purposes.
In this talk, the use of different computational tools, including quantum mechanics (QM) and molecular dynamics (MD) simulations, to reveal how mutations alter the structure and organization of enzyme active sites is presented. As initially proposed by Pauling, and elaborated by many others since then, biocatalysis is efficient when the catalytic residues in the active site of an enzyme are in optimal positions for transition state stabilization. Using MD simulations, we explore the dynamical pre-organization of the active sites of designed and evolved enzymes, by analyzing the fluctuations between active and inactive conformations normally concealed to static crystallography. MD shows how the various arrangements of active site residues influence the free energy of the transition state, and relates the populations of the catalytic conformational ensemble to enzyme activity. The understanding of how mutations modify enzymatic activities is of utmost importance to further develop new protocols to computationally design new enzymes with new and useful capabilities.
José L. Gázquez
Universidad Autónoma Metropolitana-Iztapalapa, México
Exchange energy functionals in the generalized gradient approximation
José L. Gázquez, Javier Carmona-Espíndola, Alberto Vela and S. B. Trickey
The Kohn-Sham approach of density functional theory has become one of the most useful tools to perform studies of the electronic structure of a wide variety of systems. Among the different approximations to the exchange-correlation energy functional, the generalized gradient approximation (GGA), which only makes use of the electronic density and its gradient, has been very important, because by itself it provides a good balance between accuracy and computational effort, but also because it is an important component of other approximations that incorporate additional elements, such as the kinetic energy density or a fraction of exact exchange. The GGA is usually expressed as the product of the local density approximation and an enhancement factor that is a function of the reduced density gradient, s. In a non-empirical approach, based on imposing constraints that the exact functional satisfies, the enhancement factor is approximated by interpolating between the small s and the large s limits. Although neither of those limits is uniquely defined, in this work we will focus on a small s limit that leads to a satisfactory description of properties that depend mainly on energy differences, and a large s limit that incorporates the correct asymptotic behavior of the corresponding exchange-correlation potential, and the derivative discontinuity effects, which are required for the description of properties that depend on response functions and excited states.
Peter Gill
Research School of Chemistry, Australian National University, Canberra, Australia
MP2 energies, by Jove!
Second-order Møller-Plesset perturbation (MP2) theory [1] is widely used, both in conventional wavefunction-based quantum chemistry models [2, 3] and in the more recent double-hybrid density functional procedures [4]. Its principal attraction is that it offers a rigorously based treatment of electron correlation at a cost that is not much greater than that of a self-consistent field calculation. The standard formulation of the MP2 energy involves a quadruple sum over antisymmetrized two-electron integrals divided by orbital energy differences. The formal cost of forming the two-electron integrals scales quintically with the size of the basis set. There have been several attempts to recast the sum as a numerically attractive integral. A few years ago, Willow, Kim and Hirata transformed it into a singular 13-dimensional integral which they then approximated using a Metropolis Monte-Carlo scheme [5]. Although their approach was interesting, its price-performance was not competitive with the traditional quadruple sum. They are continuing to develop this idea [6, 7]. Inspired by their work, we have recently studied an alternative recasting that yields a non-singular integral of lower-dimensionality and this appears to overcome some of the weaknesses in the Hirata approach. The new formalism, which will call Jove, offers an intriguing new pathway to MP2 energies at sub-quintic computational cost.
[1] Møller, C.; Plesset, M. S. Phys.Rev. 1934, 46, 618.
[2] Bartlett R. J.; Silver, D. M. Int.J.QuantumChem. 1974, 8, 271.
[3] Pople, J. A.; Binkley, J. S.; Seeger, R. Int.J.QuantumChem.Symp. 1976, 10, 1.
[4] Grimme, S. J.Chem.Phys. 2006, 124, 034108.
[5] Willow, S. Y.; Kim, K. S.; Hirata, S. J.Chem.Phys. 2012, 137, 204122.
[6] Willow, S. Y.; Hermes, M. R.; Kim, K. S.; Hirata, S. J.Chem.TheoryComput. 2013, 9, 4396.
[7] Willow, S. Y.; Zhang, J.; Valeev, E. F.; Hirata, S. J.Chem.Phys. 2014, 140, 031101.
Ireneusz Grabowski
Institute of Physics, Faculty of Physics, Astronomy and Informatics, Nicolaus Copernicus University in Torun, Poland
Spin-component scaled MP2 – different parametrization for different problems
The spin-component scaled second-order Møller-Plesset (SCS-MP2) methods are based on a separate scaling of the parallel and antiparallel spin components of the MP2 correlation energy expression. Because of the significant improvement over standard MP2, the SCS idea has spread out in the electronic structure method developer community and many variants have been published [For review see 1]. Unfortunately, it seems to, then there is not possible to use one set of parameters for different type of applications. Here we investigate the properties (parametrization) of the SCS-MP2, based on the correlation energy and electron density analysis. We show that the same-spin- and opposite-spin-correlated electronic densities are almost proportional to each other at each point in the real space. We also propose a modified parametrization of the scaled-opposite-spin (SOS) MP2 variant, which allow to obtain results (correlation energies, IPs, interaction energies) with almost CCSD(T) accuracy and computational cost O(N^4). Also simple and non–empirical method to determine optimal scaling coefficients, within the SCS- MP2 approach, for calculating intermolecular potential energies of non-covalently–interacting systems is presented. [2,3] Some applications of SCS-MP2 in the ab initio DFT[4,5] methodology are also discussed.
[1] S. Grimme, L. Goerigk, and R. F. Fink, Wiley Interdisciplinary Reviews:Computational Molecular Science 2, 886 (2012)
[2] I. Grabowski, E. Fabiano, F. Della Sala, Phys. Chem. Chem. Phys. 15 (37)(2013), 15485
[3] E. Fabiano, F. Della Sala, I. Grabowski, Chem. Phys. Lett. 635 (2015), 262
[4] I. Grabowski, E. Fabiano, F. Della Sala, Phys. Rev. B. 87 (2013), 075103-1
[5] S. Śmiga, F. Della Sala, A. Buksztel, I. Grabowski, E. Fabiano, J Comput Chem 37 (2016); 2081
Yuri Grin
Max-Planck- Institut für Chemische Physik fester Stoffe, Dresden, Germany
Chemical bonding in intermetallic compounds
Intermetallic compounds contain of elements left from the Zintl line in the Periodic Table. For interpretation of their chemical and physical properties (catalytic, thermoelectric, heavy-fermion behavior) a deeper understanding of the chemical composition and crystal structure is necessary. A direct link between the crystal structure as well the atomic interactions for this group of inorganic substances revels often a lack of reliable information on all three, composition, crystal structure, and bonding. Especially, chemical bonding in intermetallic compounds is a rather open question [1]. An application of quantum-chemical tools in real space like Electron Localizability approach [2] allows revealing the – on first glance unexpected - relation between organometallic and intermetallic compounds [3] or opening the way to unique definition of the important categories of chemical bond descriptors like covalence or ionicity [4] or polarity [5]. The latter gives a possibility of Zintl-like interpretation for large group of intermetallic phases. Also the formulation of the 8-N Pearson rule was derived for the real space [5,6] opening an opportunity to understand the electronic reasons for stability and crystal structure organization of the so-called Heusler phases (MgAgAs structure type) and related groups of intermetallic compounds like sphalerite family [6,7]. Bonding-caused features in the structures of TmAlB4 and YbAlB4 lead to the new magnetic interactions and remarkable difference in the magnetic properties between the ideal and real material [8,9]. In the real structure of CeIn5Ir, particular interactions between Ce and Ir result in a coexistence of two different structural patterns within one single crystal [10].
[1] Yu. Grin. In Comprehensive Inorganic Chemistry II, Vol 2. Oxford Elsevier, 2013, p. 359.
[2] M. Kohout, Int. J. Quantum Chem. 2004, 97, 651; M. Kohout, Faraday Discuss. 2007, 135, 43; F. R. Wagner et al. Chem. Eur. J. 2007, 13, 5724; A. Martin-Pendas et al. in Modern Charge-Density Analysis, Springer Heidelberg, 2012, p. 303.
[3] M. V. Butovski et al. Nature Chem. 2010, 2, 741.
[4] D. Bende at al. Chem. Eur. J. 2014, 20, 9702.
[5] D. Bende et al. Inorg. Chem. 2015, 54, 3970.
[6] F. R. Wagner et al. Dalton Trans. 2016, 45, 3236.
[7] D. Bende et al. In : Heusler Alloys – Properties, Growth, Applications, Springer, 2015, p.133.
[8] T. Mori et al. J. Appl. Phys. 2010, 107, 09E112.
[9] T. Mori et al. Phil. Mag. 2013, 93, 1054.
[10] S. Wirth et al. J. Phys. Soc. Jap. 2014, 83, 061009.
Miquel Huix-Rotllant
CNRS - Université Aix-Marseille, France
Efficient density functional methods for photochemistry
Recently, there is a growing interest in organic photochemistry. Numerous new applications appear in the literature, such as bulk heterojunction organic photovoltaics, studies of biological macromolecules (rhodopsins, DNA photodamage, cryptochromes, etc.), pump-probe experiments showing interesting new insights such as long-lived excited states and quantum coherences, or the promises of quantum control of photochemical reactions. In order to simulate such processes, wavepackets have to be propagated in several excited state potential energy surfaces exploring sometimes large phase-space regions. Thus, there is a need for efficient methods to obtain high quality potential energy surfaces and excited state properties. In this talk, I will speak about two approximate methods based on density functional theory. On the one hand, I will show how to go beyond the adiabatic approximation of the exchange-correlation kernel used in Casida's formulation of Kohn-Sham Time-Dependent Density-Functional Theory (TDDFT). This allows to obtain a description of excited states taking into account double and higher-order excitations, as well as obtaining the right topology for conical intersections between ground and excited states. On the other hand, I will show an extension of restricted ensemble Kohn-Sham (REKS) theory, in which ground and excited state interaction is included in the functional. This allow again to obtain topologically correct conical intersections, with the advantage that REKS goes beyond the single-reference KS restriction, and thus includes some multi-configuration character in the ground-state density.
Eduardo V. Ludeña
CIDNA-ESPOL, Guayaquil, Ecuador
Approximate Kinetic Energy Functionals Expressed as a Power Series of the One-Electron Density
Eduardo V. Ludeña, 1,2 Edison X. Salazar.3 Pedro Guarderas, 4 Mauricio H. Cornejo, 1 Valentin V. Karasiev, 5 Dario Arroyo. 6
1 Center of Nanotechnology Research and Development (CIDNA), ESPOL, Km 30.5 Vía Perimetral, Campus Gustavo Galindo, Guayaquil, Ecuador; 2 Centro de Química, Instituto Venezolano de Investigaciones Científicas, IVIC, Apartado 21827, Caracas, Venezuela; 3 Universidad Regional Amazónica IKIAM, Km 7 Vía Muyuna, Tena, Ecuador; 4 Universidad Central del Ecuador, Cdla. Universitaria, Quito, Ecuador; 5 Quantum Theory Project, Departments of Physics and Chemistry, P.O. Box 118435, University of Florida, Gainesville, Florida, 32611-8435, US; 6 Departamento de Física, Escuela Politécnica Nacional, Quito, Ecuador
Summary
Based on the Liu-Parr expansion (Liu and Parr, Phys Rev A 1997, 55, 1792) for the functional of the non-interacting kinetic energy, given in terms of a power series of the one-electron density, we investigate the adequateness of this approximation to describe the behavior of the kinetic energy enhancement factor for atoms from Li to Kr. It is shown that that the three-term Liu-Parr expansion when corrected locally by a term containing the Laplacian of the density - which does not modify the kinetic energy values – provides enhancement factors which approach quite closely the exact ones.[1]
In addition we apply these series to represent the Pauli kinetic energy functional and explore the effect that the size of the expansion has on the accuracy of the approximation. In particular, we examine the effect that a shell-by-shell mean-square optimization of the expansion coefficients has on the kinetic energy values. The results yield a mean absolute percentage error of 0.07, 0.04, 0.03 and 0.01 for expansions with 4, 5, 6 and 7 optimized coefficients,respectively.[2] These results are the most accurate ones as yet obtained for the representation of the non-interacting kinetic energy functional.
[1]. Edison X. Salazar,* Pedro F. Guarderas, Eduardo V. Ludeña, Mauricio H. Cornejo, Valentin V. Karasiev, Study of Some Simple Approximations to the Non-Interacting Kinetic Energy Functional, International Journal of Quantum Chemistry 2016, DOI: 10.1002/qua.25179.
[2]. Eduardo V. Ludeña, Edison X. Salazar, Mauricio H. Cornejo, Darío Arroyo, Pedro Guarderas. Representation of the Pauli kinetic energy functional in terms of the Liu-Parr power series expansion: Application to atoms. (To be submitted to The Physical Review A).
Ángel Martín Pendás
Universidad de Oviedo. Spain
Towards real space indicators of the metallic state: Partitioning the localization (or position spread) tensor.
The search for real space indicators of metallic behavior is an active field that has been lately revitalized after recognizing that the algebraic or exponential decay rate of delocalization indices may be used to differentiate conductors from insulators [1,2]. Here we analyze the Localization Tensor introduced by Kohn [3], further developed by Resta [4], and recently applied in the molecular realm by Evangelisti [5]. We show how to partition it in an origin independent manner, and relate it to previous work.
[1] A. Gallo-Bueno, A. Martín Pendás, Phys. Chem. Chem. Phys. 2016 18, 11772. [2] A. Gallo-Bueno, M. Kohout, A. Martín Pendás, submitted. [3] W. Kohn, Phys. Rev. 1964, 133, A171. [4] R. Resta, Phys. Rev. Lett. 1998, 80, 1800. [5] O. Brea et. al., J. Chem. Theory Comput. 2013, 9, 6286.
Vladimiro Mujica
Univ. Arizona, US and Donostia Int. Physics Center (DIPC)
Theoretical modeling of solar cells and photovoltaic devices
The description, control and understanding of a complex system that performs the conversion of light into a voltage generation is a complex problem that involves the use of different tools ranging from pure quantum mechanics to the theory of open systems. In this talk, I will review some of the most important ingredients of such a theoretical description including the key issues of interfacial charge and energy transport, time and length scales, the competition between charge separation and charge recombination and the most important requirements and limitations for real systems. Finally, I will examine the most relevant architectures and designs for these devices.
Juan E. Peralta
Department of Physics and Science of Advanced Materials Program, Central Michigan University, Mount Pleasant, MI 48859, US
Magnetic Exchange Couplings in Transition Metal Complexes from Density Functional Theory
I will review our recent efforts for the calculation of magnetic exchange couplings in transition metal complexes from density functional theory (DFT). I will focus first on the performance of different DFT approximations, including hybrid density functionals, and show that including approximately 30% Hartree-Fock type exchange is in general the best choice in terms of accuracy.[1,2] Second, I will describe a novel computational method to extract exchange coupling parameters using analytic self-consistent linear response theory. This method avoids the explicit evaluation of energy differences, which can become impractical for large transition metal complexes. Our approach is based on the evaluation of the transversal magnetic torque between magnetic centers using explicit constraints of the local magnetization direction via Lagrange multipliers.[3] This method can be used in combination with any modern density functional with a noncollinear spin generalization, and importantly, it can be utilized as a “black-box”. I will show proof-of-concept calculations in frustrated FeIII7 disk-shaped clusters, and dinuclear CuII, FeIII, and heterodinuclear complexes.[4,5]
[1] J. J. Phillips and J. E. Peralta, J. Chem. Phys. 2011, 134, 034108.
[2] J. J. Phillips and J. E. Peralta, J. Chem. Theory Comput. 2012, 8, 3147.
[3] J. J. Phillips and J. E. Peralta, J. Chem. Phys. 2013, 138, 174115.
[4] J. J. Phillips, J. E. Peralta, and G. Christou, J. Chem. Theory Comput. 2013, 9, 5585.
[5] J. J. Phillips and J. E. Peralta, J. Phys. Chem. A 2014, 118, 5841.
Ewa Pastorczak
Lodz University of Technology, Poland, Ecole Polytechnique Fédérale de Lausanne, Switzerland
Insight from numbers: Analyzing intramolecular non-covalent interactions with intraSAPT.
Non-covalent interactions are a vast and diverse class of phenomena impacting the behavior of all kinds of chemical systems: from the noble gas clusters, through the interaction of graphene layers to the helical structure of DNA. Computational chemistry has developed a range of tools to facilitate the quantification and analysis of those interactions: the visualization tools like NCI and DORI, which show the impacted regions of physical space, computational methods able to produce very accurate binding energies and energy decomposition analysis schemes, that decompose the interaction into physically motivated components, like exchange, electrostatic and dispersion. Among the latter, Symmetry Adapted Perturbation Theory is (SAPT)[1] prominent as a method able to both compute the interaction energies and decompose them into rigorously defined components. Such analysis is useful in rational design of materials, designing new computational methods and understanding the driving forces of chemical reactions.
Until recently, SAPT was only able to tackle intermolecular problems, which inhibited its use for problems such as catalysis reactions, stability of crowded molecules or conformations of proteins.
Here, we propose an intramolecular variant of SAPT [2,3] inspired by the Chemical Hamiltonian approach [4] and we apply it to some illustrative chemical problems, such as the conformations of hairpin alkanes, intramolecular <math>\pi</math>-<math>\pi</math> stacking or hydrogen bonding in quasi-ring structures of aminoalcohols.
[1] B. Jeziorski, R. Moszyński and K. Szalewicz, Chem. Rev., 1994 94 (7), 1887.
[2] J. F. Gonthier and C. Corminboeuf, J. Chem. Phys., 2014 140(15), 154107.
[3] E. Pastorczak, A. Prlj, J. F. Gonthier and C. Corminbœuf, J. Chem. Phys., 2015 143(22), 224107.
[4] I. Mayer, Int. J. Quant. Chem., 1983 23(2), 341.
Lucia Reining
Laboratoire des Solides Irradiés, Ecole Polytechnique, 91128 Palaiseau, France, and European Theoretical Spectroscopy Facility (ETSF)
Fingerprints of electron correlation in materials: understanding and predictions from electronic structure theory
Electronic excitations determine the characteristics of materials all over science and technology, from photochemistry to radiation defects, from synchrotron experiments to cancer research. Theory and numerical modelling are valuable tools for the understanding and prediction of many of the important phenomena. During the last decades the field has evolved rapidly, but there are still many challenges. For example, some structures in the excitation spectra of interacting electrons, called quasi-particle peaks, can be directly related to excitations of independent electrons. Others, instead, such as satellites in the photoemission spectra of solids, cannot be understood in such a simple way. They are pure consequences of interaction and correlation, and they cannot be interpreted in a pure band structure picture. First principles calculations are in general very efficient in describing band structure, especially methods based on many-body perturbation theory such as the so-called GW approximation [1]. However, they often have difficulties to describe quantitatively, or even qualitatively, everything that goes beyond. In this talk we will see what state-of-the art first principles calculations can today contribute to our understanding, focussing on the main ideas of the underlying theories, their conceptual and technical limitations, and useful comparisons with, and interpretation of, experiment. We will show ways to go beyond currently used approximations, and we will discuss fingerprints of correlation in photoemission, inelastic x-ray scattering and optical spectra, making close connections between theory and experiment. Systems used for illustration will include models, simple metals and semiconductors, carbon nanostructures and transition metal oxides [2].
The results have been obtained in collaboration with many colleagues in the Theoretical Spectroscopy Group of the Laboratoire des Solides Irradiés and in the European Theoretical Spectroscopy Facility.
[1] L. Hedin, Phys. Rev. 139, A796 (1965).
[2] see e.g. M. Guzzo et al., Phys. Rev. Lett. 107, 166401 (2011) and Phys. Rev.B 89, 085425 (2014); M. Gatti, G. Panaccione, and L. Reining, Phys. Rev. Lett. 114, 116402 (2015); J. Zhou et al., J. Chem. Phys. 143, 184109 (2015); P. Cudazzo et al., Phys. Rev. Lett. 116, 066803 (2016).
Albeiro Restrepo
Univ. Antioquia, Colombia
Dissecting reaction mechanisms
Chemical reactions are highly complex events that involve the transformation of reactants into products along one or several elementary steps. It is possible, under the Born-Oppenheimer approximation, to collapse the reaction's multidimensional potential energy surface into a continuous bidimensional profile that includes all elementary steps as a function of ξ, the reaction coordinate, which is in turn a function of all geometrical changes that define the minimum energy path connecting reactants with products. This bidimensional profile is technically known as the intrinsic reaction coordinate (IRC) and carries loads of useful information to study chemical processes. The goal of this talk is to introduce some of the tools that have been developed to extract that information.
In a general sense, it can be argued that chemical reactions are controlled by structural and electronic changes that occur along the IRC. The fundamental hypothesis is that the progress of a chemical reaction may be followed by monitoring changes in quantities that evolve in a continuous fashion along the IRC. Among other concepts, we will discuss the reaction force, the reaction force constant, the reaction electron flux, local and global reactivity indices, the evolution of breaking/formation of chemical bonds as seen from bond orders and their derivatives, orbital interactions and topological analyses of the electron densities.
Specific cases to be discussed include the nucleophilic addition step of the Michael reaction and the interconversion of the Dewar thiophene. Our results allow us to postulate that for highly complex reactions, involving multiple bond beaking/formation, the activation energy is reduced by favoring non synchronicity, that is, for this type of systems, primitive changes occur at different rates.
Tomas Rocha
Univ. Autónoma de México, México
Non-additive effects of H-bond: a quantum chemical topology perspective
The Quantum Theory of Atoms in Molecules (QTAIM)[1] and the partition of the electronic energy in accordance with the Interacting Quantum Atoms(IQA) approach[2,3] are important methods in the field of Quantum Chemical Topology. Both QTAIM and IQA have provided valuable insights about many different important systems and phenomena in chemistry. This seminar addresses the use of IQA to the analysis of cooperative[4] and anticooperative[5] effects of hydrogen bonding in small water clusters. We will also discuss the use of QTAIM along with time-dependendent density functional theory to examine the ultrafast excited state intramolecular proton transfer in salicylideneaniline, a process driven by changes in the aromaticity of the S0 and S1 states of this Schiff base.[6] Finally, we will consider the nature of resonance assisted hydrogen bonds along with the cooperative and anticooperative effects in these interactions.[7] Overall, we hope that the developments presented herein will be helpful in the understanding of the crucial non-additive effects present in H-bonds.
[1] R. F. W. Bader, Atoms in molecules: A Quantum Theory, Oxford University Press, 1990.
[2] P. Salvador, M. Duran, I. Mayer, J. Chem. Phys. 2001, 115, 1153.
[3] M. A. Blanco, Á. Martín Pendás, E. Francisco, J. Chem. Theory Comput. 2005, 1, 1096.
[4] J. M. Guevara-Vela, R. Chávez-Calvillo, M. García-Revilla, J. Hernández-Trujillo, O. Christiansen, E. Francisco, A. Martín Pendás, T. Rocha-Rinza, Chem. Eur. J. 2013, 19, 14304.
[5] J. M. Guevara-Vela, V. A. M.-G. E. Romero-Montalvo, R. Chávez-Calvillo, M. García-Revilla, E. Francisco, Á. Martín Pendás, T. Rocha-Rinza, Phys. Chem. Chem. Phys. 2016, DOI:10.1039/c6cp00763e.
[6] L. Gutiérrez-Arzaluz, F. Cortés-Guzmán, T. Rocha-Rinza, J. Peón, Phys. Chem. Chem. Phys. 2015, 17, 31608.
[7] J. M. Guevara-Vela, E. Romero-Montalvo, A. Costales, A. Martín Pendás, T. Rocha-Rinza 2016, Accepted for publication in Phys. Chem. Chem. Phys.
Pedro Salvador
Univ. de Girona, Spain
Effective Oxidation States Analysis: some applications
The concept of oxidation state (OS) is widespread in transition metal chemistry and in the study of redox and catalytic reactions. The reactivity, spin-state, spectroscopic and geometrical features of transition metal complexes are often rationalized on the basis of the oxidation state of the metal center. The IUPAC Gold Book includes a set of rules to derive OS/ON but no clear cut definition, which has paved the way for strong debates about misconceptions and inconsistencies over the last decades. Recently, prof. Karen [1,2] lead a IUPAC technical report on an unambiguous definition of OS and a set of algorithms to empirically obtain their numerical values. The report contains a wide number of examples and challenging systems for which, sometimes, the empirical oxidation state’s assignments were still ambiguous. Unfortunately, not much was mentioned in the IUPAC report about computational chemistry approaches to the problem. Oxidation states are intrinsically related to electronic distribution, but the atomic charge after the formal electron counting is only imaginary. There have been several attempts to derive OS from first principles.[3,4] In this work, we will discuss our approach to the problem, the so-called Effective Oxidation States (EOS) analysis,[5] from which OS are derived from the analysis of the first-order density matrix. The method is general and it can formally be applied to any molecular system and level of theory. It provides a set of OS assignations to all atoms or fragments defined, together with an overall index that measures to which extent the (often) integer formal charges conform with the actual electronic distribution of the molecular system.
We will present the details of the method and show some of the applications of the EOS analysis we have undertaken so far, in particular the challenging systems put forward by Karen, in order to explore the limits of applicability of the method, and to which extent the empirical algorithms can be put into correspondence with a genuine non-empirical wave function analysis approach.
1 P. Karen, P. McArdle and J. Takats Pure Appl. Chem. 2014 86(6),1017–1081
2 P. Karen, Angew. Chemie - Int. Ed. 2015, 54, 4716–4726
3 A.J. Thom, E. J. Sundstrom, M. Head-Gordon, Phys. Chem. Chem. Phys. 2009 11, 11297-11304
4 P. H-L Sit, R. Car, M.H. Cohen, and A. Selloni, Inorg. Chem. 2011, 50 10259-10267
5 E. Ramos-Cordoba, V. Postils, P. Salvador, J. Chem. Theory Comput. 2015 11, 1501–1508
Jorge Seminario
Texas A&M University, US
Lithium-Ion Behavior in and Electron leakage to the Ethylene Carbonate Electrolyte in Rechargeable Li-ion Batteries with Silicon Anodes
The performance of lithium-ion batteries is strongly dependent on the nature of the electrolyte and a better understanding of its role in ion transport and formation of solid electrolyte interface is critical for the performance improvement of such batteries. New cathode and anode materials demand new and/or improved electrolytes, which are less sensitive to operating conditions and provide higher conductivity and mobility of ions between electrodes. A clear understanding of solvation of electrolytes in solvents is essential to improve performance and life cycling of Li-ion batteries (LiB). On the other hand, understanding the molecular processes that lead to the formation, structure, and transport properties of the solid electrolyte interphase (SEI) found in lithium-ion batteries with silicon anodes is of paramount importance for the development of LiB capable of performing under the extreme exigencies of our present energy needs that are solved presently with nonrenewable energies.
The behavior of lithium hexafluorophosphate (LiPF6) in ethylene carbonate has been characterized using classical molecular dynamics simulations and ab-initio density functional theory (DFT) calculations. The solvation structure of both Li+ (Figure 1) and PF6− in the electrolyte is analyzed in detail and the intermolecular and intramolecular potentials are found to produce all the essential features of the electrolyte as observed from ab-initio DFT calculations. The thermodynamics as well as transport properties obtained using molecular dynamics simulations are also in good agreement with experimental values [1]. Then, we use a combined DFT and Green’s function approach (DFT-GF) to study the electron leakage characteristics in selected finite models of materials formed at the SEI located between the silicon surface of the anode of Li-ion batteries and the electrolyte solvent (Figure 2). The SEI products examined are lithium carbonate (LiCO3) silicon oxide (SiO2) and lithium disilicate (Li2Si2O5). Results show that the leakage of electrons from the Si anode to the solvent is greatly reduced (up to 4 orders of magnitude) with the addition and growth of the SEI components as compared with the solvent-anode sample where no SEI is present [2].
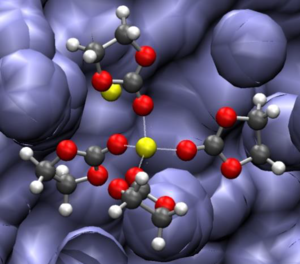
Figure 1. Li-ion (yellow) solvated with four ethylene carbonate molecules on a mixture of solvent and LiPF6 salt.
Figure 2. Calculated electron leakage from a lithiated silicon anode to ethylene carbonate solvent through a lithium carbonate interphase during charging of a LIB.
[1] N. Kumar and J. M. Seminario, "Lithium-Ion Model Behavior in an Ethylene Carbonate Electrolyte Using Molecular Dynamics," J. Phys. Chem. C, In Press.
[2] L. Benitez, D. Cristancho, J. M. Seminario, J. M. Martinez de la Hoz, and P. B. Balbuena, "Electron transfer through solid-electrolyte-interphase layers formed on Si anodes of Li-ion batteries," Electrochimica Acta, vol. 140, pp. 250-257, 2014.
Alejandro Toro-Labbe
Pont. Univ. Cat. Chile, Chile
Title
Summary
[1] Popelier, P. L. A.; Brémond, É. A. G. Int.J.Quant.Chem. 2009, 109, 2542.
Sam Trickey
University of Florida, US
Density Functionals for Atoms Under Stringent External Specifications
S.B. Trickey and Wuming Zhu
Summary
Atoms in magnetic fields characteristic of the neighborhood of neutron stars (B = 105 – 108 tesla) are examples of stringently specified systems. Finding their equilibrium properties is a difficult computational challenge which implicates the chronic tension between pursuit of chemical accuracy in functional development and the need for rapid simulations to screen large numbers of rather similar systems over wide ranges of external parameters. The usual problem of semi-local approximate functionals, self-interaction error, is compounded severely in such strongly confined systems. We have found that exact exchange combined with the TPSS correlation functional results in a self-interaction-free (XC) functional, from which we obtain atomic energies of comparable accuracy to those from correlated wave-function methods [1]. Independently, a bit later, Furness et al. used the same combination for molecules in high B [2]. Such success at high fields leads to interest in whether similar accuracy can be obtained at B=0. One of the oldest issues in that category is the failure of simple semi-local XC functionals to give properly degenerate levels with respect to the magnetic quantum number m. That issue can be framed as one of proper treatment of current density in the XC functional in the context of a strict specification of symmetry for the Kohn-Sham system. We report the complex basis methods by which we have adapted the RPA C functional to that role and the results[3].
Work supported by Zhejiang Provincial Natural Science Foundation of China grant No. LY13A050002, National Natural Science Foundation of China grants Nos. 11474081 and 11274085 (WZ) and U.S. Dept. of Energy grant DE-SC-0002139 (SBT).
[1] W. Zhu, L. Zhang, and S.B. Trickey, Phys. Rev. A 90, 022504 (2014)
[2] J.W. Furness, J. Verbeke, E.I. Tellgren, S. Stopkowicz, U. Ekström, T. Helgaker, and A.M. Teale J. Chem. Theory Comput. 11, 4169 (2015)
[3] W. Zhu, L. Zhang, and S.B. Trickey, J. Chem. Phys. (submitted)
Cyrus Umrigar
Cornell Univ.
Efficient Methods for Finding Nearly Exact Ground State Energies in Large Slater Determinant Spaces
Adam Holmes, Sandeep Sharma, Norm Tubman and Cyrus J. Umrigar
Full Configuration Interaction is feasible only for very small systems because the number of determinants grows combinatorially fast in the number of electrons and orbitals. On the other hand the number of determinants that contribute substantially to the wavefunction is a tiny fraction of the total, and perturbation theory is accurate when the zeroth order wavefunction includes the most important determinants. This talk will discuss algorithms for greatly speeding up the sampling of important determinants in a stochastic (quantum Monte Carlo) approach [1], and the selection of important determinants in a deterministic variational plus perturbation theory approach [2]. The carbon and chromium dimers are used as illustrative examples.
[1] Efficient Heat-Bath Sampling in Fock Space, Adam A. Holmes and C.J. Umrigar, J. Chem. Theory Comput. 12, 1561 (2016).
[2] Heat-Bath Configuration Interaction: An Efficient Selected Configuration Interaction Algorithm Inspired by Heat-Bath Sampling, Adam A. Holmes, Norm M. Tubman and C.J. Umrigar, J. Chem. Theory Comput. 12, 3674 (2016).
Henryk Witek
National Chiao Tung Univ., Hsinchu, Taiwan
Analytical form of helium wave function
Implicit analytical form of the ground state wave function for helium was proposed by Fock in 1954 [1]. Explicit determination of the Fock coefficients turned out to be a complex task; only few of them are found up to date [1–11]. The Fock series can be considered as an extended Taylor series around the triple coalescence point. The current talk will show how the Fock series emerges. In particular, it will show how the logarithmic terms appear in low-order Fock coefficients in order to guarantee the physical behavior (continuity and finiteness) of the resulting wave function. The questions of Fock expansions for helium in states with non-zero angular momentum and for helium without the Born-Oppenheimer approximation will also be addressed.
[1] Fock, V. A. , Norske Vidensk. Selsk. Forh. 1958, 31, 138–152 . (English translation of earlier Russian paper: Fock, V. A. Izv. Akad. Nauk 1954, 18, 161–172.)
[2] Ermolaev, A. M. Vest. Len. Univ. 1961, 16, 19–33.
[3] Abbott, P. C. PhD Thesis, Univ. Western Australia, Perth 1986.
[4] Abbott, P. C.; Maslen, E. N. J. Phys. A 1987, 20, 2043-2075.
[5] Gottschalk, J. E.; Abbott, P. C.; Maslen, E. N.; J. Phys. A 1987, 20, 2077-2104.
[6] Gottschalk, J. E.; Maslen, E. N. J. Phys. A 1987, 20, 2781-2803.
[7] Pluvinage, P. J. Physique 1982, 43, 439-458.
[8] Forrey, R. C. Phys. Rev. A 2004, 69, 022504.
[9] Liverts, E. Z. Phys. Rev. A 2014 89, 032506.
[10] Liverts, E. Z.; Barnea, N. Phys. Rev. A 2015, 92, 042512.
[11] He, B.-H.; Witek, H. A. J. Chin. Chem. Soc. 2016, 63, 69-82.
Xin Xu
Fudan University, China
Recent advances on the XYG3-type of doubly hybrid density functionals
Doubly hybrid (DH) functionals present a new class of density functionals, which enfold the non-local orbital-dependent components not only in the exchange part, but also in the correlation part. Different types of DH functionals have been proposed according to different philosophies [1-3], where the XYG3-type of functionals (xDH) [3-11] is unique in its framework that a conventional (general) Kohn-Sham (KS) functional, such as B3LYP [3-6] or PBE0 [7] or PBE [8], is utilized for the self-consistent-field (SCF) calculations to generate orbitals and density, with which a DH functional is used for the final energy evaluations.
This talk focuses on our recent efforts in the development of the xDH functionals. (1) A long-range-corrected XYG3 (i.e., lrc-XYG3) is developed, which includes a range-dependent term from the second order perturbation theory for better description of dispersive interaction [6]. (2) Analytic gradients are developed, where the non-variational contributions from the SCF functional to the final energy functional are solved through a coupled-perturbed KS equation [9]. (3) Fractional charge behaviours of DH functionals are explored [10-12], which lead to good predictions of ionization potentials, electron affinities and fundamental gaps from the perspective of fractional charges. (4) A non-fitted DH functional, namely PBE-ACDH, is constructed based on the adiabatic connection (AC) formalism, coordinate scaling relations, and the second order Görling-Levy perturbation theory [8], where contributions from density scaling and singles are explicitly considered.
Limitations of the present approaches and the direction for future improvements will be discussed.
This research was sponsored by the Ministry of Science and Technology of China (2013CB834606, 2011CB808505), and National Natural Science Foundation of China (21133004, 91427301).
[1] Y. Zhao, B. J. Lynch and D. G. Truhlar, J. Phys. Chem. A 108, 4786 (2004).
[2] S. Grimme, J. Chem. Phys. 124, 034108 (2006).
[3] I. Y. Zhang, X. Xu, and W. A. Goddard III, Proc. Nat. Acad. Sci, USA, 106, 4963 (2009).
[4] I. Y. Zhang, X. Xu, Y. Jung, and W. A. Goddard III, Proc. Nat. Acad. Sci, USA, 108, 19896 (2011).
[5] I. Y. Zhang, X. Xu, Int. Rev. Phys. Chem. 30, 115(2011).
[6] I. Y. Zhang, X. Xu, J. Phys. Chem. Letters, 4, 1669 (2013).
[7] I. Y. Zhang, N. Q. Su, É. A. G. Brémond, C. Adamo, X. Xu, J. Chem. Phys. 136, 174102 (2012).
[8] N. Q. Su, X. Xu, J. Chem. Phys., 140, 18A512 (2014).
[9] N. Q. Su, I. Y. Zhang, X. Xu, J. Comput. Chem., 34, 1759 (2013).
[10] N. Q. Su, W. T. Yang, P. Mori-Sánchez, X. Xu, J. Phys. Chem. A 118, 9201 (2014).
[11] N. Q. Su, X. Xu, J. Chem. Theory Comput.11:4677 (2015).
[12] N. Q. Su and X. Xu, J. Chem. Theory Comput.(2016) DOI:10.1021/acs.jctc.6b00197.
Weitao Yang
Department of Chemistry and Physics, Duke University, Durham, N.C. 27708, U.S.A.
Going Beyond Conventional Functionals with Local Scaling Corrections and Pairing Fluctuations in DFT
Fractional fractional charges and fractional spins provide a clear analysis of the errors of commonly used functionals. For an effective and universal alleviation of the delocalization error, we develop a local scaling correction scheme by imposing the Perdew-Parr-Levy- Balduz linearity condition to local regions of a system. Our novel scheme is applicable to various mainstream density functional approximations. It substantially reduces the delocalization error, as exemplified by the significantly improved description of dissociating molecules, transition-state species, and charge-transfer systems. The usefulness of our novel scheme affirms that the explicit treatment of fractional electron distributions is essentially important for reducing the intrinsic delocalization error associated with approximate density functionals.
Progress with many-body theory approach will be also be presented. We have formulated the ground-state exchange-correlation energy in terms of pairing matrix linear fluctuations, opening new a channel for density functional approximations. This method has many highly desirable properties. It has minimal delocalization error with a nearly linear energy behavior for systems with fractional charges, describes van der Waals interactions similarly and thermodynamic properties significantly better than the conventional RPA, and eliminates static correlation error for single bond systems. It is the first known functional with closed-form dependence on orbitals, which captures the energy derivative discontinuity in strongly correlated systems. We also adopted pp-RPA to approximate the pairing matrix fluctuation and then determine excitation energies by the differences of two-electron addition/removal energies. This approach captures all types of interesting excitations: single and double excitations are described accurately, Rydberg excitations are in good agreement with experimental data and CT excitations display correct 1/R dependence. Applications to the singlet-triplet energy gaps of diradicals and poly-acenes and conical intersections will be featured.
References
[1] A.J. Cohen, P. Mori-Sanchez, and W. T. Yang. Challenges for Density Functional Theory. Chem. Rev. 112:289, 2012
[2] X. Zheng, A. J. Cohen, P. Mori-Sanchez, X. Q. Hu, and W. T. Yang. Improving band gap prediction in density functional theory from molecules to solids. Physical Review Letters, 107(2):026403, 2011.
[3] Chen Li, Xiao Zheng, Aron J. Cohen, Paula Mori-Sánchez, and Weitao Yang, Local Scaling Correction for Reducing Delocalization Error in Density Functional Approximations Physical Review Letters 114, 053001.2015
[4] H. van Aggelen, Y. Yang, and W. T. Yang. Exchange-correlation energy from pairing matrix fluctuation and the particle-particle random-phase approximation. Physical Review A, 88(3):030501, 2013.
[5] Y. Yang, H. van Aggelen, and W. T. Yang. Double, Rydberg and charge transfer excitations from pairing matrix fluctuation and particle-particle random phase approximation. Journal of Chemical Physics, 139:224105, 2013.
[6] Y. Yang, D. G. Peng, J. F. Lu, and W. T. Yang. Excitation energies from particle-particle random phase approximation: Davidson algorithm and benchmark studies. Journal of Chemical Physics, 141:124104, 2014.
[7] Yang Yang, Lin Shen, Du Zhang and Weitao Yang, Conical Intersections from Particle–Particle Random Phase and Tamm–Dancoff Approximations, J. Phys. Chem. Lett. 7, pp 2407–2411, 2016
[8] Yang Yang, Ernest R. Davidson, and Weitao Yang, Nature of ground and electronic excited states of higher acenes, Proceeding of the National Academy of Sciences, early edition, 2016 [doi: 10.1073/pnas.1606021113
Dominika Zgid
Univ. Michigan, US
Title
Summary
[1] Popelier, P. L. A.; Brémond, É. A. G. Int.J.Quant.Chem. 2009, 109, 2542.
Posters
Grids are 1.2 m wide x 2.4 m high. Poster size is recommended of 1m wide x 1.5 m high.
Please add abstracts in Surname alphabetical order
Elmer Gastelo
Universidad Nacional de Ingenieria, Lima. Perú
Photocatalytic activity of cobalt ferrite CoFe2O4-nanoparticles subjected to heat treatment
Cobalt ferrite nanoparticles synthetized by the sol-gel method [1] were obtained by iron salts FeCl2.4H2O and cobalt Co(NO3)2.6H2O as precursors, using cetyltrimethylammonium bromide (CTAB) as a surfactant. These were subjected to heat treatment for 3.4hours[2] at different temperatures 200°C, 275°C, 300°C, 350°C, 400°C, 550°C and 750°C. Subsequently these were characterized by X-ray diffraction (XRD), field emission scanning electron microscopy (FE-SEM) and photocatalytic activity on bacteria Escherichia coli strain ATCC 22922. The results XRD show diffractograms own cobalt ferrite for samples how obtained and calcined (200°C-550°C) but a phase change temperature on 750°C was observed, using the formula Debye-Scherrer has calculated the grain size of the particle obtained sizes 18.7± 3.3 nm for the sample how obtained and 22.8 ± 2.0 nm, 21.2±1.9nm, 19.2±1.4nm, 24.2±1.5nm, 23.8±2.0nm, 22.2±1.90nm, 68.8±13.1nm for the samples calcined at 200°C, 275°C, 300°C, 350°C, 400°C, 550°C and 750°C respectively. The morphology of the samples were studied by FE-SEM images, which show spherical nanoparticles with average size of 27.0±8.0nm, 29.4±14.5nm, 58.9±30.4nm, 47.1±26.0nm, 70.3±39.4nm, 35.9±24.7nm by samples calcined at 200°C, 275ºC, 300ºC, 350ºC, 400ºC and 550ºC respectively, for the sample calcined at 750°C morphology was shaped nanoflakes with a size of 190.7±61.1nm. Finally, the photocatalytic activity of the samples was studied to disinfection of bacteria Escherichia coli in water [3].
[1] Juan M. de Oca, Ll. Chuquisengo, H. Alarcón, 2010. Síntesis y caracterización de nanopartículas de ferrita de cobalto obtenidas por el proceso sol-gel, Revista Sociedad Química Perú 76(4), 400-406. [2] Sajjia, M., Benyounis, K.Y., Olabi, A.G., 2012. The simulation and optimization of heat treatment of cobalt ferrite nanoparticles prepared by the sol–gel technique. Powder Technology 222, 143–151. [3] Casbeer, E., Sharma, V.K., Li, X.-Z., 2012. Synthesis and photocatalytic activity of ferrites under visible light: A review. Separation and Purification Technology 87, 1–14. ↑ top of this page
María Luisa Cerón
Universidad Finis Terrae. Escuela de Ingeniería. Av. Pedro de Valdivia 1509, Providencia, Santiago. Chile
Application of Dual Descriptor to understand the activity of Cu/ZrO2 catalysts in the water gas shift reaction
A comparative theoretical study applying Dual Descriptor[1] has been performed using Water Gas Shift reaction[2], catalyzed by copper supported on monoclinic and tetragonal zirconia. The Dual Descriptor and thermodynamic considerations were used to understand the structural effects that occur in the reaction when it is catalyzed by Cu on both zirconia phases. For these systems were found that the formation of carbonates in the Cu supported on tetragonal zirconia the presence of carbonates necessary for the decomposition of the formates allowing the dehydrogenation this effect is consistently with experimental results[3].
1. C. Morell, A. Grand, A. Toro-Labbé, J. Phys. Chem. A. 2005, 109, 205.
2. M. Rhodes, A. Bell, J. Catal. 2005, 233, 198.
3. G. Águila, S. Guerrero, P. Araya, Catal. Comm., 2008, 9, 2550.
↑ top of this page
Eduardo Chamorro
Universidad Andres Bello. Departamento de Ciencias Químicas. Facultad de Ciencias Exactas. 8370146 Santiago. Chile
Theoretical scales of electrophilicity and nucleophilicity.
The suitability of intrinsic (i.e., electronic) relative indices for quantifying electrophilicity and nucleophilicity responses [1-4] is critically examined. Theoretical results are discussed within the framework of experimental reactivity categorization based on the linear free energy methodology developed by Mayr and coworkers [5-9]. The polar nucleophilic/electrophilic activation (as measured through simple descriptors) is shown to be a key factor driving the initial rate-determining steps of the electrophile-nucleophile coupling.
[1] Chamorro, E.; Melin, J. On the Intrinsic Reactivity Index for Electrophilicity/Nucleophilicity Responses. J. Mol. Mod. 2015, 21. DOI: 10.1007/s00894-015-2608-2.
[2] Chamorro, E.; Duque-Norena, M.; Notario, R.; Perez, P. Intrinsic Relative Scales of Electrophilicity and Nucleophilicity. J. Phys. Chem. A. 2013, 117, 2636-2643.
[3] Chamorro, E.; Duque-Norena, M.; Perez, P. A Comparison between Theoretical and Experimental Models of Electrophilicity and Nucleophilicity. J. Mol. Struct.-Theochem. 2009, 896, 73-79.
[4] Chamorro, E.; Duque-Norena, M.; Perez, P. Further Relationships between Theoretical and Experimental Models of Electrophilicity and Nucleophilicity. J. Mol. Struct.-Theochem. 2009, 901, 145-152.
[5] Mayr, H.; Ofial, A. R. A Quantitative Approach to Polar Organic Reactivity. SAR QSAR Environ. Res. 2015, 26, 619-646.
[6] Mayr, H. Reactivity Scales for Quantifying Polar Organic Reactivity: The Benzhydrylium Methodology. Tetrahedron. 2015, 71, 5095-5111.
[7] Mayr, H.; Ofial, A. R. Do General Nucleophilicity Scales Exist? J. Phys. Org. Chem. 2008, 21, 584-595.
[8] Mayr, H.; Ofial, A. R. Kinetics of Electrophile-Nucleophile Combinations: A General Approach to Polar Organic Reactivity. Pure Appl. Chem. 2005, 77, 1807-1821.
[9] Mayr, H.; Patz, M. Scales of Nucleophilicity and Electrophilicity - a System for Ordering Polar Organic and Organometallic Reactions. Angewandte Chemie-International Edition in English. 1994, 33, 938-957.
Ricardo Inostroza Rivera
Facultad de Ciencias de la Salud, Universidad Arturo Prat, Casilla 121, 1110939 Iquique, Chile
The Beckmann Rearrangement in the Framework of Reaction Electronic Flux
We have computationally investigated the mechanism of Beckmann rearrangement (BR) [1] in the framework of Reaction Electronic Flux [2-3]. The reaction has been studied in three different reaction conditions. The electronic transfer contribution of the reaction electronic flux was found to play a crucial role in this reaction. Natural bond order analysis and dual descriptor [4] provide additional support for elucidating the mechanism of this reaction.
[1] E. Beckmann Chem. Ber., 1886, 89, 988.
[2] S. Vogt-Geisse, A. Toro-Labbé. J. Chem. Phys., 2009, 130, 244308.
[3] R. Inostroza-Rivera, B. Herrera, A. Toro-Labbé. Phys. Chem. Chem. Phys., 2014, 16, 14489.
[4] C. Morell, A. Grand, A. Toro-Labbé, J. Phys. Chem. A. 2005, 109, 205.
Macarena Muñoz
University of Chile
How predictive could alchemical derivatives be?
One of the main challenges in materials science is the rational design of compounds, i.e., establishing experimental and theoretical protocols for the design of materials with properties optimized for specific applications. From the theoretical point of view, and in particular electronic structure, the challenge is enormous. To ilustrate this it is sufficient to note the vastness of the "chemical space" [1-3], that is, the set of plausible stable compounds that can be made with elements of the periodic table. Conservative estimations of only a subset of possible small organic molecules lead to the conclusion that this space contains much more than 10⁶⁰ compounds [4]. This work shows that alchemical transformations [5,6] are efficient alternative to explore the energetic landscape of chemical space. Specifically, we show that alchemical derivatives can be used as an efficient screening of the potential stable isomers of the Al13-nSin cluster. We reveal the importance of “electron transfer” effects by comparing isoelectronic and non-isoelectronic alchemical transformations.
[1] P. Kirkpatrick and C. Ellis, Nature 432, 823 (2004).
[2] C. M. Dobson, Nature 432, 824 (2004).
[3] O. A. Von Lilienfeld and M. E. Tuckerman. The Journal of Chemical Physics 125, 154104 (2006).
[4] R. S. Bohacek, C. McMartin, and W. C. Guida, Medicinal Research Reviews 16, 3 (1996).
[5] O. Anatole von Lilienfeld, The Journal of Chemical Physics 131, 164102 (2009).
[6] M. to Baben, J. O. Achenbach, and O. A. von Lilienfeld. The Journal of Chemical Physics 144, 104103 (2016).
Wilver A. Muriel
National University of Colombia, Medellín
Molecular Dynamic Study Of The Excited State Intermolecular Proton Transfer Of 2-Salicylideneantrhylamine In The First Excited Stated
The main challenge for designing photo-active material is to understand processes that follow after light is absorbed. When a molecule absorbs light, both the distribution of the electrons and nuclei change This causes changes in the physical and chemical properties of the molecule, such as potential energy, molecular geometry, polarizability, charge distribution, etc.. Understanding these processes is essential when designing photo-active materials. This poster presents a study of the intramolecular proton transfer in the first excited state of the 2-salicylideneanthrylamine. In order to elucidate dynamic processes in the molecule in question we have used time-dependent density functional theory (TD-DFT) molecular dynamics.
Patricia Perez
Universidad Andres Bello. Departamento de Ciencias Químicas. Facultad de Ciencias Exactas. 8370146 Santiago. Chile
Carbenoid-type reactivity of nitrile ylides in [3+2] cycloaddition reactions towards electron-deficient ethylenes
The [3+2] cycloaddition (32CA) reaction of nitrile ylide (NY) with an electron-deficient ethylene is studied at the MPWB1K/6-31G(d) computational level. A structural analysis of NY shows a carbenoid (cb) structure, allowing its participation in cb-type 32CA reactions. In gas phase, this 32CA reaction is not stereoselective and has low regioselectivity. Inclusion of solvent effects does not modify the activation energy, but increases the meta regioselectivity in clear agreement with the experimental outcomes. Electron localisation function (ELF) topological analysis for the formation of the two C-C single bonds along the four competitive channels associated with this 32CA reaction makes it possible to characterise two dissimilar mechanisms.
1. L.R. Domingo, M. Ríos-Gutiérrez, M. Duque-Noreña, E. Chamorro, P. Pérez.
Theoret. Chem. Acc., 2016, 135,160.
Osvaldo Yañez
Doctorado en Fisicoquímica Molecular, Universidad Andrés Bello, República #275, Santiago-Chile
A Simple Hybrid Algorithm for Exploring the Potential Energy Surface of Clusters and Molecules
The development of optimization strategies (local and global optimization) is a subject of great importance in diverse fields, including the physical sciences (for example, protein folding and atomistic structural determination of macro-molecules and clusters). The main difficulty is that the global extremum of a real multivariate function is actually a local property, thus requiring an exhaustive search. Furthermore, proving that the global extremum has indeed been found seems to be a rather unattainable task for most systems of interest.
There were several algorithms proposed for solving this problem. The most popular ones being simulated annealing1, genetic algorithms2, and particle swarm optimization3. While all these methods were effective for calculation of relatively small molecules, they proved to be ineffective when we tried to deal with larger molecules containing more than 12 atoms. The main objective of this work is to build larger atomic clusters using molecular fragments. For this purpose, we implemented a modification to the Kick program developed by Addicoat and Metha4 and Steepest Descent algorithm; by using adequate molecular fragments confined in arbitrarily selected spaces. The performance of this approximation was evaluated in the theoretical design of Lithium-Silicon nanowires, using the most stable isomers of the previously reported Si5Li6 cluster. The Si-Li nanomaterials are important for technological applications in ion-Li batteries for increasing their energy storage capability.
Acknowledgements:
The author thanks CONICYT for a Doctoral fellowship.
[1] Kirkpatrick, S.; Gelatt, C. D.; Vecchi, M. P. Optimization by Simulated Annealing. Science (80-. ). 1983, 220 (4598), 671–680.
[2] Alexandrova, A. N.; Boldyrev, A. I. Search of the Lin 0/+1/-1 (n=5-7) Lowest-Energy Structures Using the Ab-Initio Gradient Embedded Genetic Algorithm (GEGA). Elucidation of the Chemical Bonding in the Lithium Clusters. J. Chem. Theory Comput. 2005, 1, 566.
[3] Call, S. T.; Zubarev, D. Y.; Boldyrev, A. I. Global Minimum Structure Searches via Particle Swarm Optimization. J. Comput. Chem. 2007, 28 (7), 1177–1186.
[4] Addicoat, M. A.; Metha, G. F. Kick: Constraining a Stochastic Search Procedure with Molecular Fragments. J. Comput. Chem. 2009, 30 (1), 57–64.566.
Alejandro Vásquez Espinal
Doctorado en Fisicoquímica Molecular, Universidad Andrés Bello, República #275, Santiago-Chile
Departamento de Física, Facultad de Ciencias Exactas, Universidad Andres Bello, República #220, Santiago, Chile
Departamento de Química, Facultad de Ciencias Exactas, Universidad Andres Bello, República #275, Santiago, Chile
Star-Shaped clusters as building blocks in the design of Nanowires
We explored the viability of using star shaped clusters1 based on elements of groups 1 and 14 as assembly units to build nanowires. Firstly, we simulated the structure of the dimers of the assembled blocks to see if the star-shaped structure persisted at a molecular level. In order to do this, we performed different searches in the potential energy surface of the dimers using the Gradient Embedded Genetic Algorithm (GEGA)2 and Born Oppenheimer Molecular Dynamics3 simulate the collision of two monomers. Secondly, we proposed adequate building blocks (unit cells) to build nanowires, using the spatial arrangements of the atoms of the stabilized dimers simulated before as a reference.
Preliminary results showed that, in the case of the silicon-lithium nanowires the position and orientation of the assembly unit is determinant on the electronic properties. For example, when the blocks are put together in an eclipsed way the obtained nanowire exhibits a metallic character, while when the blocks are put in an alternate way, the obtained nanowire is an insulator with a band gap of 2.8 eV.
[1] W. Tiznado, N. Perez-Peralta, R. Islas, A. Toro-Labbe, J. M. Ugalde and G. Merino, J. Am. Chem. Soc., 2009, 131, 9426-9431.
[2] A. N. Alexandrova and A. I. Boldyrev, Journal of Chemical Theory and Computation, 2005, 1,566-580.
[3] J. M. Millam, V. r. Bakken, W. Chen, W. L. Hase and H. B. Schlegel, The Journal of Chemical Physics, 1999, 111, 3800-3805.
Ricardo Pino Rios
Doctorado en Fisicoquímica Molecular, Universidad Andrés Bello, República #275, Santiago-Chile
Evaluating the predictive capability for hydrogen bonds: Topological vs Magnetic approaches
The hydrogen bond plays an important role in chemical, physical and biological processes1 . Its characterization has led to a number of both theoretical and experimental investigations. According to the energetic and spectroscopic criteria, there are three kinds of hydrogen bond types: Weak, medium and strong. In this work, we evaluate the predictive capability of the strength of a hydrogen bond by two different criteria. The first one is the topological approach based on the integration of popular non-covalent interactions (NCI) index2 , which has been widely applied qualitatively in the study of these systems. The second one is the magnetic criteria based on a recent study of Elgabarty et. al.3 , who designed a theoretical model based on the magnetic anisotropy in order to study the degree of covalency of the hydrogen bonds in liquid water.
A test of small systems comprising three types of hydrogen bonds was applied Energy results were obtained by second order perturbation theory calculations. Preliminary results indicate that both methods appropriately predict the strength of the hydrogen bonds studied; however, which of these models is the most appropriate in the description?
[1] Grabowski, S., Hydrogen Bonding - New Insights. Springer Netherlands: 2006.
[2] (a) Contreras-García, J.; Yang, W.; Johnson, E. R., Analysis of Hydrogen-Bond Interaction Potentials from the Electron Density: Integration of Noncovalent Interaction Regions. The Journal of Physical Chemistry A 2011, 115 (45), 12983-12990; (b) Johnson, E. R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A. J.; Yang, W., Revealing Noncovalent Interactions. Journal of the American Chemical Society 2010, 132 (18), 6498-6506.
[3] Elgabarty, H.; Khaliullin, R. Z.; Kuhne, T. D., Covalency of hydrogen bonds in liquid water can be probed by proton nuclear magnetic resonance experiments. Nat Commun 2015, 6.
Rodrigo Báez Grez
Doctorado en Fisicoquímica Molecular, Universidad Andrés Bello, República #275, Santiago-Chile
Quantitative estimation of aromaticity in Azines
Due to the vast amount of aromaticity analysis with inconsistent findings throughout the azine series 1-3, this work is proposed as it follows; a simple method to compare and quantitatively estimate the aromaticity. This method uses as a basis, the theory of NMR to map the magnetic field induced in the z component.
Each of the azine molecules, is positioned independently, in the center a grid ranging from -4 to +4 Å in the xy plane with a width of 0.2 Å, as is done for nucleus-independent chemical shifts (NICS)4. The results are displayed in line graphs, an alternative proposed in the work of Kleinpeter et al.5 in which the criterion of aromaticity is given by the height of the graphic. 6
[1] Wiberg, K. B.; Nakaji, D.; Breneman, C. M. J. Am. Chem. Soc. 1989,111, 4178.
[2] See Bird’s series of papers on “heteroaromaticity”, e.g.: (a) Bird, C. W. Tetrahedron 1992, 48, 335. (b) Bird, C. W. Tetrahedron 1996, 52, 9945. (c) Bird, C. W. Tetrahedron 1997, 53, 13111.
[3] (a) Mandado, M.; Otero, N.; Mosquera, R. A. Tetrahedron2006,62, 12204. (b) Mandado, M.; Gonzalez, M. J.; Mosquera, R. A. J. Comput.Chem. 2007, 28, 127.
[4] P. v. R. Schleyer, H. Jiao, N. J. R. v. E. Hommes, V. G. Malkin, O. L. Malkina.J. Am. Chem. Soc. 1997, 119, 12669.
[5] Klod, S.; Kleinpeter, E. J. Chem. Soc., Perkin Trans. 22001, 1893−1898.
[6] Carion, R.;Liégeois, V.; Champagne, B.; Bonifazi, D.; Pelloni, S.; Lazzeretti, P. J. Phys. Chem. Lett.2010, 1, 1563–1568.
Edison Osorio
Departamento de Ciencias Básicas, Fundación Universitaria Luis Amigó, SISCO, Transversal 51A #67B 90, Medellín, Colombia
Stabilization of Al3C2Hn (n =1-6) carbides: transformation from two to three dimensions toward two structures with planar tetracoordinates carbons.
The metal carbide clusters have been extensively studied as a new class of materials for use in semiconductors, ceramics, hydrogen storage, and catalysis. Into this research area, the aluminum carbides are considered as non-classical and non-stoichiometric systems, with a different form of the most metal carbide clusters. Experimental and theoretical studies have shown that the lowest energy structures for Al2C2, Al3C2, Al4C2, Al5C2 and Al6C2 clusters are stabilized due to the presence of C–C fragment, however for hydrogenated Al2C2Hx (x =1-8) systems the theoretical studies have shown that the lowest energy structures with C-C fragment are conserved only until Al2C2H4.1 Due to the conduct of C-C fragment for stabilizing the Al2C2 cluster and the hydrogenated, we are interested in understanding if the C-C portion could stabilize the next series of hydrogenated aluminum carbides, Al3C2Hx (x =1-6). For this propose, we performance a stochastic research on the potential energy surface for Al2C3Hx (x =1-6) using GEGA algorithm,2 showed interesting results. The first correspond to an evolution in the lowest structures from 2 to 3 dimension, where the C-C fragment only is present for Al2C3H1 and Al2C3H2 systems; the second results correspond to the localizing of two molecules composed with planar tetracoordinate carbons (ptC), global minima for Al2C3H1 and Al2C3H2 systems.3,4 Finally, in this work the stabilities and evolution for these systems are explained in terms of chemical bonding analysis.
[1] F. Dong, S. Heinbuch, Y. Xie, J. J. Rocca and E. R. Bernstein, Phys. Chem. Chem. Phys., 2010, 12, 2569–2581.
[2] A. N. Alexandrova and A. I. Boldyrev, J. Chem. Theory Comput., 2005, 1, 566–580.
[3] J. Zhu, B. Wang, J. Liu, H. Chen and W. Zhang, RSC Adv., 2015, 5, 13923–13929.
[4] Z. Cui, C. Shao, S. Gao and Y. Ding, Phys. Chem. Chem. Phys., 2010, 12, 13637–13645.
Franklin Ferraro
Departamento de Ciencias Básicas, Fundación Universitaria Luis Amigó, SISCO, Transversal 51A #67B 90, Medellín, Colombia
Instituto de Química, Universidad de Antioquia, Colombia
Exploration of the usefulness of a Gold-Platinum Cluster as a Catalyst for the Dehydrogenation of Ammonia-Borane
Due to its ability to store large amounts of hydrogen (19.6 wt.%), the ammonia borane molecule (AB = H3N-BH3) is considered one of the main alternatives of new energy source. One of the problems to be resolved about this substance is finding suitable methods to extract its hydrogen. Among some others, catalytic methods are a very good alternative.1,2
In this work we explore the use of the predicted more reactive gold-platinum tetramer cluster, the global minimum species of composition Au2Pt2 , 3 as a catalyst for the dehydrogenation of AB. A systematic stochastic-based search 4 and subsequent gradient- based optimization of the AB-Au2Pt2 structures, including relativistic effects for the Au and Pt atoms, gave an exhaustive scanning of the configurational space for the system. It was found that the AB molecule always interact with the Au2Pt2 clusters through the platinum atoms. This result is the first step to understand the catalytic activation process and the consequent migration of atomic hydrogen in the cluster. After making structural and electronic characterizations of this system, additional stochastic searches were made with two and three molecules of ammonia borane using different methodologies to explore the possibility of hydrogen release.
[1] H-L. Jiang, Q. Xu. Catal. Today, 2011, 170, 56. <br\>
[2] Z-H. Lu, Q. Yao, Z. Zhang, Y. Yang, X. Chen. J. Nanomater., 2014, 2014, 4. <br\>
[3] N. Moreno, F. Ferraro, E. Florez, C. Z. Hadad, A. Restrepo J. Phys. Chem. A,2016, 120, 1698. <br\>
[4] J. F. Pérez, C. Z. Hadad, A. Restrepo, Int. J. Quantum Chem., 2008, 108, 1653. <br\>
Dayán Páez Hernández
Facultad de Ciencias Exactas,ReMoPhys, Universidad Andrés Bello, República 275, Santiago, Chile
Magnetic Properties of 4f and 5f Elements. A Theoretical View
SO-CASPT2 is a first principle two step method is method which makes first spin free calculations and spin-orbit coupling in the second step The weak field approximation, which is the most suitable for situations where spin-orbit effects dominate interactions with ligands, the total angular momentum J is a good quantum number and we first describe the ground state in terms of |J, MJ > states, where MJ is the projection of J over the main axe of the molecule. Let us first consider the effect of the spin-orbit interaction on the f1 configuration. This removes the degeneracy of the 2S+1L = 2F ground state and gives two states 2F5/2 and 2F7/2 characterized by J =5/2 and 7/2, respectively. By other hand the metal-ligand interactions remove the 2J + 1 degeneracy of 2F5/2 and 2F7/2 states to doubly degenerate Kramers states and introduce the second-order mixing of states with different J and the same MJ value. In the following we considered both effects to describe the magnetic properties of lanthanide and actinide complexes.
Keywords. Actinide, magnetic properties
Acknowledgements:
The author acknowledge funding from Grant Nos. FONDECYT 11140295 and Millennium Nucleus RC120001.
[1] Kahn, O. Molecular Magnetism.; Wiley-VCH: New York., 1993.<br\>
[2] Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions.; Clarendon Press: Oxford.,1971.<br\>
Walter A. Rabanal-León
Universidad Nacional Andrés Bello, Facultad de Ciencias Exactas, Departamento de Ciencias Químicas, Av. República 498, Santiago, Chile
Revisiting the Aromatic Character of the [Re3(μ-Cl)3Cl6]2- According Magnetic and Electron Density Criteria
In this contribution, the behavior of the [Re3(μ-Cl)3Cl6]2- cluster under a magnetic perturbation was theoretically evaluated, and it clearly shows that the magnetic response within the Re3(μ-Cl)3 plane is highly diatropic in nature. An analysis of both the magnetically induced current density (MICD) and induced magnetic field (Bind) allows us to classify this cluster as doubly σ- and also π-aromatic on the magnetic criterion. These findings contradict the classical Re−Re double bond representation and favor a chemical bonding pattern that involves delocalized bonds. Additionally, the use of tools based on the electron density and the topological analysis of this was used. These topological analyses based on the localization of the electron density (ELF, ELI-D, QTAIM, multicenter index) could not provide significant information about aromaticity when these schemes are used separately. Nevertheless, a complementary used of them could support the existence of an aromatic behavior at least in a qualitatively way.
Acknowledgements:
W.A.R.L. acknowledges CONICYT for his post-doctoral project FONDECYT / Postdoctorado - 2016 No 3160388.
References:
[1] Alvarado-Soto, L.; Ramírez-Tagle, R.; Arratia-Pérez,R. J. Phys. Chem. A 2009, 113, 1671-1673.
[2] Sergeeva, A. P.; Boldyrev, A. I. Comments Inorg. Chem. 2010, 31, 2-12.
[3] Alejandro Vásquez-Espinal, Ricardo Pino-Rios, Luis Alvarez-Thon, Walter A. Rabanal-León, Juan J. Torres-Vega, Ramiro Arratia-Pérez, and William Tiznado. J. Phys. Chem. Lett. 2015, 6, 4326-4330
Karen Palacio
Química de Recursos Energéticos y Medio Ambiente, Instituto de Química, Facultad de Ciencias Exactas y Naturales, Universidad de Antioquia, UdeA-Colombia, Calle 70 No. 52-21, Medellín, Colombia.
Theoretical Study of Thiol-Michael Addition Reactions: Substituent Effects on Reactivity of Aurones with Thiols of Biological Interest
Michael addition reactions of flavonoids (aurones) with biothiols, like cysteine were evaluated from thermodynamic and kinetic points of view. The Electronic properties of aurones (Michael acceptors) and cysteine (Michael donor) have been studied used Density Functional Theory1. Some global and local descriptors of reactivity like electrophilicity, electronic chemical potential, and chemical hardness have been calculated in order to describe the reactivity of each aurone in accordance to their substituents.
The mechanism of the Michael addition were simulated using PBE0 hybrid functional (also known as PBE1PBE) and 6-31+g(d) basis set. The solvent effects (water) in activation energy were included using the polarisable continuum model (PCM). It was found that the transition state of the rate-determining step is a simultaneous four-centre addition of the neutral thiol across the C=C double bond, according to other theoretical and experimental studies 2,3
References:
[1] Parr, R. G., & Pearson, R. G. (1983). Absolute hardness: companion parameter to absolute electronegativity. Journal of the American Chemical Society,105(26), 7512-7516
[2] Schwöbel, J. A. H., Madden, J. C., & Cronin, M. T. D. (2010). Examination of Michael addition reactivity towards glutathione by transition-state calculations.SAR and QSAR in Environmental Research, 21(7-8), 693-710.
[3] Aliaga, M. E., Tiznado, W., Cassels, B. K., Nunez, M. T., Millán, D., Pérez, E. G., García-Beltrán, O. & Pavez, P. (2014). Substituent effects on reactivity of 3-cinnamoylcoumarins with thiols of biological interest. RSC Advances, 4(2), 697-704.
Ronald Ruíz C.
Escuela Profesional de Química. Universidad Nacional Federico Villarreal. Lima-Perú.
Theoretical study of electronic properties in mono substituted benzoquinones.
The effects they have on the quinones depending on the type of substituent affect their reactivity and optical properties. The quinones act as dienophiles in reactions of Diels Alder 1. Through theoretical calculations using the Density Functional and a bases set of 6-31G, was determined its reactivity through global descriptors (hardness, nucleophicility, chemical potential, electronegativity and electrophilicity) 2,3 as local (Fukui indexes) 3 as well as the absorption that have these substituted molecular systems in the visible-range of the electromagnetic spectrum.
It was found a scale of electrophilicity/nucleophicility in these quinones according to the substituent similar to previous studies proposed 4; demonstrating a reciprocity between the nucleophicility and electrophilicity that works for these systems, also proposed in studies already done in others similar systems 5; at the same time it was obtained a proportional relationship between the other global descriptors such as the chemical potential, this all gives us valuable information on the reactivity that have these quinones by the type of substituent as for example in the reactions of Diels Alder, and in addition to this it was obtained a similar effect between global descriptors and the spectra of UV visible by the substituent. It was also obtained density graphs of local indexes of reactivity that complements the obtained results where influences the generated electronic delocalization 6.
References:
[1] R. H. Thomson. Naturally Occurring Quinones. Department of Chemistry, University of Aberdeen, Scotland. Academic Press. (1971).
[2] Kohn, W.; Becke, A. D.; Parr, R. G. J. Phys. Chem. (1996), 100, 12974.
[3] P. Geerlings, F. De Proft, and W. Langenaeker. Conceptual Density Functional Theory. Chem. Rev. (2003), 103, 1793 – 1873.
[4] S. Lakhdar, M. Westermaier, F. Terrier, R. Goumont, T. Boubaker, A. R. Ofial and H. Mayr, J.Org.Chem., (2006),71, 9088.
[5] Parr, R. G.; Szentpály, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922.
[6] Halina Szatyzowicz. Routes of π - Electron Delocalization in 4-Substituded -1, 2 –benzoquinone. J. Org. Chem. Vol. 76, No. 2, (2011). 550 – 556.
Victor Garcia
Doctorado en Fisicoquímica Molecular, Universidad Andrés Bello, República #275, Santiago-Chile
Theoretical study of inhibitor reactivity of tau protein
The development of tau aggregation inhibitors could be a potential alternative as a treatment strategy for neurofibrillary tangles produced from tau deposit formation in Alzheimer´s disease.1 In this theoretical study, we analyzed structure-activity relationships of five series of 12 substituted rhodanines, four substituted phenothiazines, two substituted of N-phenylamines, one of thiazole and one of benzothiazole inhibitors. The analysis was performed to confront inhibitory activity (IC50) according to experimental results reported by Bulic et al. with DFT global and local reactivity descriptors. 2-5 The rhodanine and N-phenylamines cores have been reported as a carboxylic bioisoster by size, low electronegativity and ability to engage hydrogen bonds, but also the substitution patterns are important since they determine the total length of the molecule. 5 The aim of this work is to find an appropriate theoretical model that explain those latest reported experimental results.
References:
[1] Bulic, B., Pickhardt, M., Khlistunova, I., Biernat, J., Mandelkow, Eva-Maria., Mandelkow, E.,Waldmann, H.,Angew. Chem. 2007, (46), 9215-9219. (1971).
[2] Wischik, C., Harrington, C., Storey, J., Biochemical Pharmacology 2014 (88) 529-539.
[3] Geerlings, P., Del Proft, F., Langenaeker, W.,Chem. Rev. 2003 (103) 1793-1873.
[4] Van Damme, S. Bultinick, P.,J. Comput. Chem. 2009 (30) 1749-1757
[5] Bulic, B., Pickhardt, M., Mandelkow, Eva-Maria Mandelkow., Neuropharmacology 2010 (59) 276-289.
Desmond MacLeod Carey
Universidad Autónoma de Chile, Santiago, Chile
Mixed-Valence Polyoxomelate Dumbbell
Matías Reyes López,1 Dayán Páez Hernández,1 Desmond MacLeod Carey2
1 Universidad Andres Bello, Centro de Nanociencias Aplicadas (CENAP), Doctorado de Fisicoquímica Molecular, Departamento de Ciencias Químicas, Facultad de Ciencias Exactas, Av. República 275, Santiago, Chile.
2 Universidad Autónoma de Chile, Inorganic Chemistry and Molecular Materials Center, Instituto de Ciencias Químicas Aplicadas, Facultad de Ingeniera, El Llano Subercaseaux 2801, San Miguel, Santiago, Chile.
Theoretical and experimental aspects about the electronic transference in molecular systems containing one or more redox centers have received considerable attention in last decades, especially those where exist a strong interaction between two or more redox centers, in which, the electron transfer of these "mixed valence systems" has been studied since long time ago.[1] Into this context, Robin and Day propose a classification according to the degree of interaction between two redox centers from an essential null interaction (Class I), a moderated one (Class II) towards a very strong electronic coupling (Class III).[2]
In the present work, we carry out a serie of theoretical calculations with the aim to understand the molecular and electronic structure of a serie of Inorganic-Organic Hybrid Molecular Dumbbells constituted by two Lindqvist type Polyoxometalate units bridged with a organic spacer, see Figure 1. We analyze the effect of the enlargement of the organic spacer over their molecular and electronic structure of their mixed valence species obtained through the reduction and oxidation of them. We have found that in reduced mixed valence species, the extra electron is mainly located over the polyoxometalate unit, while that in oxidized mixed valence species, the organic spacer behaves as a non-innocent ligand, since participates as a redox center within the molecule. Thus, the oxidized mixed valence species can be classified in the border line of Class II-III according to the electronic interaction of the Polyoxometalate units.
Figure 1. a) Molecular structure of a dumbbell system. b) Spin density of mixed-valence oxidized species. c) Spin density of mixed-valence reduced species.
Keywords. Polyoxometalates, Functionalization, Mixed-Valence
Acknowledgements:
The author acknowledge funding from Grants FONDECYT 1131123 and Millennium Nucleus RC120001.
References
[1] a) M.H.V. Huynh, T.J. Meyer, Chem. Rev. 2007, 107, 5004
b) K.D. Demadis, C.M. Hartshorn, T.J. Meyer, Chem. Rev. 2001, 101, 2655
c) P. Piotrowiak, Chem. Soc. Rev. 1999, 28, 143
d) M.A. Fox, Chem. Rev. 1992, 92, 365
e) A.C. Benniston, A. Harriman, Chem. Soc. Rev. 2006, 35, 169
f) J-P. Launay, Chem. Soc. Rev. 2001, 30, 386
[2] M.B. Robin, P. Day, Adv. Inorg. Chem. Radiochem. 1967, 10, 247