Training for the NCI workshop
<<< Topological Approaches to Intermolecular Interactions workshop main page
| This is a special session organised by R Chaudret
THE DIFFERENT TRAINING SUPPORTS (PRESENTATION, EXERCICES AND CODES) WILL BE ADDED ON INTERNET SHORTLY. SEE THE SECTION TRAINING IN DOWNLOAD "WORKSHOP MATERIAL". |
| Practical trainings will be organized on the prior morning and/or afternoon to the workshop.
The following training activities are envisaged:
|
PARTICIPANTS TO THE TRAINING SESSION: Click on the link to acces a map of the UPMC
http://doodle.com/exzhkiegergybmd9
Speakers of the training session, please add your abstract below:
- first : log in;
- click on "edit", on the righthand corner above the the line below "Your name"
- copy the example given (also available >>> here) and paste it at the end of this page
- fill in the fields with your data
- >>> How to insert a picture in your abstract
- Don't forget to send us an email with the type of contribution you would like to make
NCI (basics) : first steps into NCIPLOT and VMD
Robin Chaudret / Julia Contreras-Garcia / Marie-Laure Bonnet
NCI (Non-Covalent Interactions) is a visualization index based on the density and its derivatives. It enables identification of non-covalent interactions. It is based on the peaks that appear in the reduced density gradient (RDG) at low densities.
There is a crucial change in the RDG at the critical points in between molecules due to the annihilation of the density gradient at these points. When we plot the RDG as a function of the density across a molecule, we see that the main difference between the monomer (Figure 1a) and dimer (Figure 1b) cases is the appearance of steep peaks at low density.
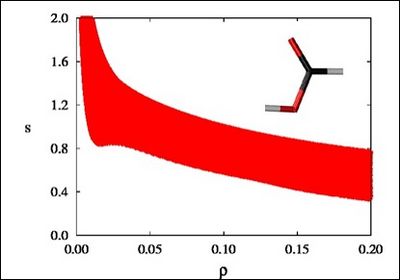
Figure 1a |
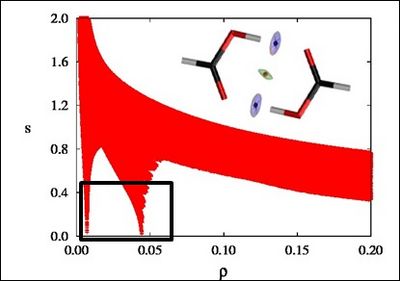
Figure 1b |
When we search for the points in 3D space giving rise to these peaks, non covalent regions clearly appear in the (supra)molecular complex (insets in Figure 1a and Figure 1b).
During this training, we will review how to carry out NCI calculations, both from promolecular and SCF calculations. We will also go into the VMD script, so that everybody knows how to visualize their output!
ELF (basics) : first steps into TOPMOD and topological analysis
Robin Chaudret / Julia Contreras-Garcia / Marie-Laure Bonnet
Coupling ELF and NCI
Robin Chaudret / Julia Contreras-Garcia / Marie-Laure Bonnet
In this final training of the morning session we will emphase on the complementarity of ELF and NCI approaches and particularly to study reaction mechanisms. Indeed, since ELF focuses on strong density area and nci on weak density ones, these approaches allow to follow the modification of the system at any step of the reaction. During the training we will specifically learn how to understand an ELF/NCI picture and to use different scripts to generate automatically ELF/NCI short movie.
Averaged NCI: using NCI for dynamic systems
Robin Chaudret
To apply the NCI analysis to fluctuating environments as in the solution phase, we developed a new averaged noncovalent interaction (i.e., aNCI) index along with a fluctuation index to characterize the magnitude of interactions and fluctuations. We implemented them into the NCIplot programm and tested them on several solute/solvant but also substrate/enzyme systems. During the training session we will rapidly review the theory of aNCI and how and why we decided to go from NCI to aNCI. Then we will do practical exercices to learn the usage of the specific NCIplot keywords going along with an aNCI calculation. We will also review several scripts that allow to create aNCI input.
NCI for solids
Alberto Otero-de-la-Roza
Chemistry and Chemical Biology, School of Natural Sciences, University of California, Merced, 5200 North Lake Road, Merced, California 95343, USA
In this training session I will give a brief introduction to critic2, a program for the analysis of the electron density (and related scalar fields) in real space. Critic2 can be used for atoms-in-molecules (QTAIM) analyses, graphical representation, arithmetic manipulation of scalar fields, crystal format conversion, and more. In this session, the primary focus will be the calculation of the non-covalent interactions (NCI) index in periodic solids. The basic procedure involves computing the electron density of the solid using any of the codes in the literature (at this moment, critic2 supports directly wien2k, elk, Quantum ESPRESSO, VASP, abinit, gaussian, and aiPI but any code that generates the electron density on a grid can be used) and then writing a simple input file that is run through critic2. The promolecular density can be used as well when the proper self-consistent density is not available. Critic2 is fully-documented and distributed under a free (GPL) license.
NCI from experimental densities
Gabriele Saleh
Department of Chemistry, Università degli Studi di Milano, via C.Golgi 19, 20159 Milano Italy
NCImilano: a code to apply non-covalent interactions descriptor to experimental and theoretical electron density distribution
The code “NCImilano” [1] is designed to apply the RDG-based NCI descriptor introduced by Johnson et al. [2] to Electron Density (ED) distribution obtained either from theoretical calculation (on both isolated molecules and crystals) or from X-ray diffraction experiments [3,4]. More specifically, it can read the output files of GAUSSIAN 03/09, TOPOND (which is interfaced to CRYSTAL 98/06/09) and XD2006. When files in XD2006 format are given in input to “NCImilano”, output files in the same format are produced, so that they can be read from the graphical routine of XD. Besides calculating Reduced Density Gradient (RDG) and ED*sign(λ2) (where λ2 is the second greatest eigenvalue of the ED Hessian matrix), i.e. the quantities needed to apply the NCI descriptor in its original formulation, this program can also produce grid files of total energy density along with its potential and kinetic contribution. The latter quantities can be evaluated either exactly from the wavefunction or, in the case of experimentally-derived ED (i.e. when the wavefunction is not available), by exploiting the approximate functional introduced by Abramov [5]. In addition, NCImilano compute the volume of RDG isosurfaces and the integral of several quantities over the space enclosed into such isosurfaces. In this talk the main features of the code NCImilano will be presented, with special emphasis on the calculation of properties from experimentally-derived ED.
References [1] G. Saleh, C. Gatti, L. Lo Presti, D. Ceresoli. Submitted to J. Appl. Cryst. on 15th March 2013 [2] E. R. Johnson, et al. (2010) J. Am. Chem. Soc. 132, 6498–6506 [3] G. Saleh, C. Gatti, L. Lo Presti, J. Contreras-Garcìa (2012) Chem. Eur. J. 18, 15523-15536 [4] G. Saleh, C. Gatti, L. Lo Presti, L. (2012) Comput. Theor. Chem. 998,148–163 [5] Y. A. Abramov (1997) Acta Cryst. A53, 264-272
Maximum Probability Domains (MPDs)
Benoit Braida, Marcos Menéndez
In the last few years it has been proposed to analyze arbitrary wave functions by searching the regions of space for which the probability to find a given number of electrons, ν, is maximal.[1-4] These regions of space, and by extension the associated interpretative method, are thus called «maximum probability domains» (MPDs). When ν = 2, it relates to the Lewis’ concept of electron pairs, and thus provides a natural connection between quantum mechanics and the traditional way of thinking of chemists (Lewis structures). In addition to the simplicity and clarity of the concept and related quantites, another significant advantage of this method is that the search for MPDs is local, so the computational cost scales moderately with systems size, which opens room for applications on large systems.
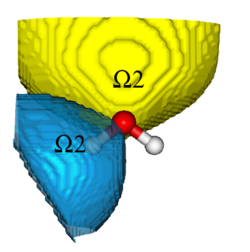
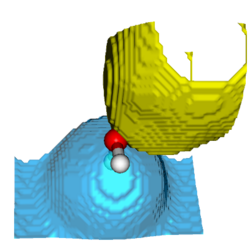
The MPD method and related concepts will be introduced, differences and advantages towards other related interpretative methods highlighted. The MPD program will be presented, and the tutorial participants will have the opportunity to use it on simple illustrative systems.
References
- Savin A (2002) In: Sen KD (ed) Reviews of modern quantum chemistry: a celebration of the 252 contributions of Robert G. Parr. World Scientific, Singapore, p43
- Cancès E, Keriven R, Lodier F, Savin A (2004) Theor Chem Acc 111, 373
- Scemama A, Caffarel M, Savin A (2007) J Comp Chem 28, 442
- Mafra Lopez Jr. O, Braïda B, Causa M, Savin A in Progress in Theoretical Chemistry and Physics vol 22, p173 ed Hoggan, Springer UK, London (2011)
- (a) Causa M, Savin A Z. Anorg. Allg. Chem. 2011, 637 ; (b) Causa M, Savin A J. Phys. Chem. A 2011, 115, 13139