Abstracts of the CTTC 2019
<<< CTTC 2019 workshop main page
Slides available after the workshop
HOW TO UPLOAD YOUR ABSTRACT
In order to upload your abstract, you will need a valid login account.
Please contact us email and we will email you your login details.
Please be aware that the whole system has been reinitialized, so old accounts are not valid anymore.
CONTRIBUTORS: please add below, in your own section, your title talk and abstract :
- first : log in; (contact the organization for log in details or use the ones we provided in 2014 if you attended Vietnam)
- click on your name in the "Contents" box below, this will lead you to your own section;
- your section starts with your name as the title line, click on [edit] (far right).
- >>> How to insert a picture in your abstract
- INVITED SPEAKERS: Please upload your abstracts before 30st April 2019.
- POSTERS: If you wish to contributethe with a poster, feel free to follow the prescribed template in the Poster section . Please upload your abstracts before 30th April 2019.
All students are strongly encouraged to present a poster at the conference.
Frederik Tielens
Vrije Universiteit Brussel Faculteit Wetenschappen en Bio-ingenieurswetenschappen Pleinlaan 2, B-1050 Brussel, Belgium frederik.tielens@vub.be
Characterization of Self-Assembled Monolayers on Noble Metal Surfaces
Self-assembled monolayers (SAMs) consist of a layer of functionalized long-chain molecules tethered to a solid substrate. SAMs have attracted significant interest of both the fundamental and applied scientific communities. Their presence as a “coating” on a surface is attractive in a number of applications due to the possibility to provide tuning of the surface properties by selectively modifying functional groups on the SAM. Alkanethiols (CH3(CH2)nSH) and alkylthiolate radicals (CH3(CH2)nS∙) adsorption on Au(111) surface is one of the most studied and best-known SAM systems, but also other bioorganic molecules such as amino acids organize at the surface. The nature of the corresponding structure at the surface has been controversial for a long time, as well as other aspects such as the adsorption site on which the thiol chain is anchored, and if the thiol adsorbs by S-H bond breaking process or not. Experimental studies shed some light on both questions, indicating that surface thiol species are attached to Au adatoms, rather than Au atoms in such a bulk-terminated layer, and that it is the movement of these Au-adatom-thiolate moieties that order to produce the SAM structure. Still, some questions remain unsolved such as: At which coverage does this happen, and for which chain length? What is the influence of the presence of defect sites (vacancy and adatoms) on the S-H bond breaking process? The thiols adsorb in a laying down geometry at low coverage, but at which coverage do they straighten up or stand up? In this context we will show here a series of results on the characterization of alkyl thiol SAMs investigated in detail by means of periodic density functional calculations.
References I. Lorenzo Geada, I. Petit, M. Sulpizi and F. Tielens, Surf.Sci. 677 (2018) 271. E. Colombo, G. Belletti, F. Tielens, P. Quaino, Appl. Surf. Sci. 452, (2018) 141. S. Kumar Meena, C. Goldmann, D. Nassoko, M. Seydou, T. Marchandier, S. Moldovan, O. Ersen, F. Ribot, C. Chanéac, C. Sanchez, D. Portehault, F. Tielens, M. Sulpizi, ACS Nano, 11, 7371 (2017). D. Nassoko, M. Seydou, C. Goldmann, C. Chanéac, C. Sanchez, D. Portehault, F. Tielens, Materials Today Chemistry 5, 34, (2017). C. Goldmann, F. Ribot, L.F. Peiretti, P. Quaino, F. Tielens, C. Sanchez, C. Chanéac, D. Portehault, Small, 13, 1604028, (2017) H. Guesmi, N. Luque, E. Santos, F. Tielens, Chem.Eur.J, 23, 1402, (2017). D. Costa, C.-M. Pradier, F. Tielens, L. Savio, Surface Science Reports, 70, 449 (2015).
Sam Trickey
Dept. of Physics and Quantum Theory Project, Univ. of Florida
Less is More – or – Back to Kohn-Sham
Simplification of widely used meta-generalized-gradient approximation (mGGA) exchange-correlation functionals by removal of their explicit orbital dependence is valuable because it re-incorporates mGGA calculations in the Kohn-Sham framework. Returning to the pure Kohn-Sham local potential framework (rather than the generalized K-S approach almost always used with orbital-dependent mGGA functionals) aids interpretability and improves computational efficiency in large-scale simulations. The talk will summarize how the Laplacian level of refinement can be achieved by use of suitably constructed approximate kinetic energy density functionals (KEDFs). The existence of Laplacian-level deorbitalizations which yield better performance than the original mGGA will be illustrated for molecules with the meta-GGA-made-very-simple functional. Results on standard molecular and condensed-phase test sets obtained from the deorbitalized version of the SCAN functional (“SCAN-L” for SCAN with Laplacian) reproduce the original SCAN error patterns rather well. However, the magnetization of bcc Fe is quite different, an important distinction that will be discussed.
Supported by U.S. Dept. of Energy grants DE-SC 0002139 and DE-SC 0019330.
References D. Mejía-Rodríguez and S.B. Trickey, Phys. Rev. B 98, 115161 (2018); Phys. Rev. A 96, 052512 (2017).
Adrienn Ruzsinszky
Department of Physics, Temple University, Philadelphia, PA 19122, USA
Going beyond the random phase approximation for materials
The Random Phase Approximation (RPA) has become a standard method beyond semilocal Density Functional Theory (DFT) that naturally incorporates weak interactions and eliminates self-interaction error in the exchange energy [1]. RPA is not completely perfect, however, and suffers from self- correlation error as well as an incorrect description of short-ranged correlation. To improve upon RPA, various beyond-RPA approximations were developed in the past decade. The most familiar approximations are the RPA+ [2], SOSEX (second-order screened exchange) [3] along with model exchange-correlation kernels within the time-dependent DFT framework [4]. Exchange-correlation kernels can be applied to excited states and optical spectra [4]. In this talk I will reveal the strengths and limitations of these methods. In addition, I will introduce new alternative routes which can deliver further improvement beyond the already existing techniques [5,6]. Applications will be also discussed.
Work is supported by NSF DMR–1553022 and DOE BES DE-SC0012575.
References:[1] H. Eshuis, J. E. Bates, F. Furche, Theor. Chem. Acc., 131, 1084 (2012) [2] S. Kurth and J. P. Perdew, Phys. Rev. B, 59, 10461, (1999) [3] A. Grüneis, M. Marsman, J. Harl, L. Schimka, and G. Kresse, J. Chem. Phys. 131, 154115 (2009) [4] J.E. Bates, S. Laricchia, and A. Ruzsinszky, Phys. Rev. B 93, 045119, (2016) [5] T. Gould, J. P. Perdew and A. Ruzsinszky, to be submitted [6] P.D. Mezei, A. Ruzsinszky, to be submitted.
Luis Rincon
Universidad San Francisco de Quito, Quito, Ecuador
The information content of the Fermi and Coulomb holes
This presentation summarizes two recently proposed information quantities which are employed to visualize the Fermi and Coulomb holes in the real space. The first one is the information content of the Exchange-Correlation hole, calculated from the Kullback–Leibler divergence of the same-spin conditional pair density respect to the marginal probability (χXC). As reported, χXC, can be used to reveal the regions of the space associated to the classical electron pair model [1-3]. The second one is the information content of the correlation hole, which is computed in terms of the Kullback–Leibler divergence of a correlated same-spin conditional pair density respect to the uncorrelated Hartree–Fock pair density (χC) [4-5]. These two information quantities are discussed on the light of the results for high-spin clusters of alkali metals.
1. L. Rincon, R. Almeida, P. L. Contreras and F.J. Torres “The information content of the conditional pair probability” Chem. Phys. Lett. 635, 116 (2015). 2. A.S. Urbina, F.J. Torres and L. Rincon “The electron localization as the information content of the conditional pair probability” J. Chem. Phys. 144, 244104 (2016). 3. L. Rincon, F.J. Torres and R. Almeida “Is the Pauli exclusion the origin of electron localization?” Mol. Phys. 116, 518 (2018) 4. L. Rincon, F.J. Torres, M. Becerra, S. Liu, A. Fritsch and R. Almeida “On the separation of the information content of the Fermi and Coulomb holes and their influence on the electronic properties of molecular systems” Mol. Phys. 117, 610 (2019) 5. F.J. Torres, L. Rincon, C. Zambrano, J.R. Mora and M.A. Mendez “A review on the information content of the pair density as a tool for the description of the electronic properties of molecular systems” Int. J. Quantum Chem. 119, e25763 (2019)
Ángel Martín Pendás
Dpto. Química Física y Analítica. Universidad de Oviedo. Oviedo, Spain
Should charge-shift bonding be reconsidered?
Charge-shift bonding (CSB) was introduced as a distinct third family of electron-pair links that adds to the covalent and ionic tradition. However, the full battery of orbital invariant tools provided by modern real space artillery shows that it is difficult to find CSB signatures outside the original valence-bond framework in which CSB was developed. Here we show that this concept should probably be further investigated.
References J. Luis Casals-Sainz, F. Jiménez-Grávalos, E. Francisco, A. Martín Pendás, Chem. Commun. (2019), DOI: 10.1039/C9CC02123J
Dennis R. Salahub
Department of Chemistry, Centre for Molecular Simulation, Institute for Quantum Science and Technology, Quantum Alberta, University of Calgary, Canada
Towards free-energy profiles for nano-catalyzed chemical reactions in complex environments
I will review our attempts to build somewhat realistic models of nanocatalysis at finite temperature. Current thoughts are to bring in machine-learning techniques to, ideally, define the relevant reaction coordinates/collective variables. Significant progress has been made on such questions in the bio- modeling literature and I would like to understand the new ML methodologies better and to, hopefully, adapt them to the field of nanocatalysis. I am a neophyte, eager for any guidance that CTTC participants might offer, once I have exposed my state of knowledge/ignorance.
Josep M. Luis
University of Girona
Density Functional Theory and Nonlinear Optical Properties
The design of new molecular materials with large nonlinear optical properties (NLOP) remains a challenging task for computational chemistry due to the necessity of accurately computing both electronic and vibrational hyperpolarizabilities of individual molecules as well as the effects of intermolecular interactions. Density Functional Theory (DFT) has proven to be a powerful tool for solving various quantum mechanical problems in a cost-effective way, but DFT performance in the area of NLOP has been under active assessment. We have performed an extensive study of the performance of a diverse set of density functional approximations in predicting the NLOP of hydrogen-bonded complexes using the CCSD(T)/aug-cc-pVTZ level of theory as reference.[1] For all the studied properties, the average absolute errors below 20% can only be obtained using the CAM-B3LYP functional, while LC-BLYP and MN15 are shown to be only slightly less accurate. We reported huge errors in predicting the vibrational second hyperpolarizability by B97X, M06 and M06-2X functionals. This large failure is traced down to a poor determination of third- and fourth-order energy derivatives with respect to normal modes. These results reveal serious flaws of some DFT methods and suggest caution in selecting the appropriate functional to calculate any molecular property that contains vibrational anharmonic contributions.
We have also analyzed the optimal tuning of range-separatiod LC-BLYP functional based on adjusting the attenuating function parameters to satisfy ionization potential theorem. Our results revealed that this approach does not bring any systematic improvement in the predictions of NLOPs. However, we have explored new strategies to tune the range-separation parameter to provide a correct description of NLOP, performing an exhaustive study of the dependency of this parameter in terms of simply quantities.[2] We have found a simple expressions for the optimal value of the attenuating parameter in terms of the second hyperpolarizability values computed at LC-BLYP level that reproduce the CCSD(T) second hyperpolarizabilities. The hyperpolarizabilities obtained with our NLOP-tailored new optimal tuned LC-BLYP are more accurate than the ones obtained with CAM-B3LYP and LC-BLYP functionals. .
References [1] Robert Zalesny, Miroslav Medved,Sebastian Sitkiewicz, Eduard Matito, Josep M. Luis, “Can Density Functional Theory Be Trusted for High-Order Electric Properties? The Case of Hydrogen-Bonded Complexes”, J. Chem. Theory Comput., submitted. [2] Pau Besalú, Sebastian Sitkiewicz, Pedro Salvador, Eduard Matito, Josep M. Luis, in preparation.
Joakim Halldin Stenlid
Department of Physics, Stockholm University, Sweden
Chemical interaction behaviour probed by the local electron attachment energy
The ability to make swift and reliable predictions of chemical interaction behavior and reactivity lies at the heart of theoretical chemistry. This presentation will examine the performance of a new DFT-based ground-state property, the local electron attachment energy [E(r)] [1], for the prediction of global and local electrophilicity/Lewis acidity. E(r) is a property based on a multi-orbital analysis of the unoccupied KS-DFT electronic states. It is found that this joint-orbital picture outperforms the frontier molecular orbital approach (FMO) for predictions of both regioselectivity and intermolecular reactivity trends. E(r) furthermore complements the electrostatic potential probe by reflecting also local contributions to charge-transfer and polarization. Examples of the use of E(r) are taken from molecular reaction theory including conjugate addition and aromatic substitution, as well as from materials science with emphasis on heterogeneous catalysis of transition metal and transition metal oxide nanoparticles and surfaces [2]. Comparisons are made to both experimental and computational data. Future directions for the efficient screening of new catalytic materials based on the new property will be discussed.
References
[1] T Brinck, P Carlqvist, JH Stenlid, Local Electron Attachment Energy and Its Use for Predicting Nucleophilic Reactions and Halogen Bonding, The Journal of Physical Chemistry A 120, 10023-10032, 2016 [2] T Brinck, JH Stenlid, The Molecular Surface Property Approach: A Guide to Chemical Interactions in Chemistry, Medicine, and Material Science, Advanced Theory and Simulations, 2, 1800149, 2019
Juan E. Peralta
Department of Physics, Central Michigan University, Mount Pleasant MI 49959
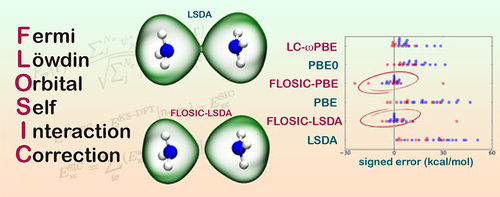
The FLO-SIC route to self-interaction-free DFT
The effect of DFT self-interaction error (SIE) on calculated molecular and solid-state properties has been known for a long time. The most widely accepted framework for removing SIE in DFT is due to Perdew and Zunger (PZ) [1]. However, due to the high computational cost associated with minimizing the PZ energy expression, the calculation of explicitly self-interaction free molecular properties remains elusive. Recently, an efficient implementation for SIE removal based on Fermi orbitals was proposed [2]. This method explicitly avoids the unitary transformation from canonical to localized orbitals that is needed in standard PZ, and replaces it by a Fermi-Löwdin transformation which depends only on one vector descriptor per orbital, or Fermi orbital descriptor (FOD). The Fermi-Löwdin orbital self-interaction correction (FLO-SIC) provides an computationally efficient alternative to the traditional PZ methods. [2] I will describe the FLO-SIC methodology and its advantages and caveats compared to PZ-SIC. I will show our recent efforts to make FLO-SIC more efficient and user-friendly. [3] As an illustration of the capabilities of the FLO-SIC method, I will show some recent results of diverse properties in cases where SIC is important and discuss the opportunities to move FLO-SIC forward.[4-6]
References
[1] J. P. Perdew and A. Zunger, Phys. Rev. B 23, 5048 (1981).
[2] M. R. Pederson, A. Ruzsinszky, and J. P. Perdew, J. Chem. Phys. 140, 121103 (2014).
[3] K. Trepte, S. Schwalbe, T. Hahn, J. Kortus, D. Kao, Y. Yamamoto, T. Baruah, R. R. Zope , K. P. K. Withanage, J. E. Peralta, and, K. A. Jackson, J. Comp. Chem. 40, 820 (2019).
[4] K. P. K. Withanage, K. Trepte, J. E. Peralta, T. Baruah, R. Zope, and Koblar A. Jackson, J. Chem. Theory Comput. 14, 4122 (2018).
[5] R. P. Joshi, K. Trepte, K. P. K. Withanage, K. Sharkas, Y. Yamamoto, L. Basurto, R. R. Zope, T. Baruah, K. A. Jackson, and J. E. Peralta, J. Chem. Phys. 149, 164101 (2018).
[6] K. Sharkas, L. Li, K. Trepte, K. P. K. Withanage, R. P. Joshi, R. R. Zope, T. Baruah, J. K. Johnson, K. A. Jackson, and J. E. Peralta, J. Phys. Chem. A 122, 9307 (2018).
Emmanuel Fromager
Université de Strasbourg, Laboratoire de Chimie Quantique, Institut Le Bel, 4 rue Blaise Pascal, 67000 Strasbourg, FRANCE
Density matrix functional embedding theory based on the Householder transformation
In the increasingly popular density matrix embedding theory (DMET) [1], a fragment (also referred to as impurity) of the full electronic system of interest is embedded into an effective reduced-in-size environment (referred to as bath). A Schrödinger-like equation can then be solved accurately (if not exactly) for the embedded cluster (impurity+bath). Despite the name of the method, the embedding procedure of standard DMET essentially relies on a state-specific and approximate (mean-field) wavefunction. Consequently, its improvement as well as its extension to finite temperatures is not straightforward.
In this talk, I will present an in-principle-exact DMET-like approach where the embedding is fully driven by the (one-electron reduced) density matrix. In such a density matrix functional embedding theory (DMFET), the bath is determined by applying a Householder transformation to the density matrix [2]. The method is in principle systematically improvable since electron correlation in the (so-called Householder) cluster environment is described by a functional of the density matrix. Exact and approximate single-impurity formulations of DMFET will be presented for the one-dimensional Hubbard model. Extensions to multiple impurities and connections with standard DMET will also be discussed.
References
[1] G. Knizia and G. K.-L. Chan, Phys. Rev. Lett. 109, 186404 (2012). [2] M. Saubanère, L. Mazouin, M. Tsuchiizu, and E. Fromager, in preparation (2019).
Daniel Finkelstein-Shapiro
Department of Chemical Physics, Lund University, Sweden
Constructing effective operators for open quantum systems using ancillary continua
Open quantum system master equations implicitly capture the effect an environment or bath (e.g. vibrational modes of a molecule, protein, solvent) on a central system of interest (e.g. electronic levels of a molecule) in the form of effective transitions that account for energy exchange between system and bath. This shifts the problem from calculating explicitly the nuclear degrees of freedom – a prohibitively expensive problem computationally – to that of finding accurate master equations. Equally important is the invaluable insight provided by simplified master equations in the form of analytically solvable toy models.
In this talk we present the solution to a family of toy models where one or more of the levels has been replaced by a continuum. These models with Hamiltonians with discrete-continuous spectrum capture real systems such as molecules on surfaces, and have a mathematical structure that affords a more transparent understanding of effective operators for open quantum systems. The solution to these problems is in the form of a nonlinear Liouville equation that describes a partition of the system that can exchange not only energy but also particle density with a generalized environment (including other levels of the electronic manifold). We use this formalism to classify the approximations used in adiabatic elimination techniques and improve them, and to formally connect the phenomenon of particle decay and Markovian dissipation.
References
[1] Finkelstein-Shapiro, D.; Urdaneta, I.; Calatayud-Antonino, M.; Atabek, O.; Mujica, V.; Keller, A. Fano-Liouville Spectral Signatures in Open Quantum Systems. Phys. Rev. Lett., 2015, 115, 113006 [2] Finkelstein-Shapiro, D.; Calatayud-Antonino, M.; Atabek, O.; Mujica, V.; Keller, A. Nonlinear Fano Interferences in Open Quantum Systems: an Exact Solution. Phys. Rev. A, 2016, 93, 063414 [3] Finkelstein-Shapiro, D.; Keller, A. Ubiquity of Beutler-Fano profiles: from scattering to dissipative processes. Phys. Rev. A. 2018, 97, 023411
Thibault Terencio
School of Chemical Science and Engineering, Yachay Tech University, Yachay City of Knowledge, 100650 Urcuqui, Ecuador.
Chemoselectivity in the oxidation of cycloalkenes with a non-heme iron(IV)-oxo-chloride complex: Epoxidation vs. hydroxylation selectivity
We report and analyze chemoselectivity in the gas phase reactions of cycloalkenes (cyclohexene, cycloheptene, cis-cyclooctene, 1,4-cyclohexadiene) with a non-heme iron(IV)-oxo complex [(PyTACN)Fe(O)(Cl)]+, which models the active species in iron dependent halogenases. Unlike in the halogenases, we did not observe any chlorination of the substrate. However, we observed two other reaction pathways – allylic hydrogen atom transfer (HAT) and alkene epoxidation. The HAT is clearly preferred in the case of 1,4-cyclohexadiene, both pathways have comparable reaction rates in reaction with cyclohexene, and epoxidation is strongly favored in reactions with cycloheptene and cis-cyclooctene. This preference for epoxidation differs from the reactivity of iron(IV)-oxo complexes in the condensed phase, where HAT usually prevails. To understand the observed selectivity, we analyze 4 different factors : effects of the substrate, effect of the complex conformation, spin state, and solvation. Our DFT and CASPT2 calculations suggest that all the reactions occur on the quintet potential energy surface. The DFT-calculated energies of the transition states for the epoxidation and hydroxylation pathways explain the observed chemoselectivity. The SMD implicit solvation model predicts the relative increase of the epoxidation barriers with solvent polarity, which explains the clear preference of HAT in the condensed phase. Finally, this study allows us to propose a strategy for the design of iron(IV)oxo complex that would be selective for hydroxylation over epoxidation.
References
Mark E. Tuckerman
Department of Chemistry and Courant Institute of Mathematical Science, New York University
Molecular simulation and Machine Learning as Routes to Exploring Structure and Phase Behavior in Atomic and Molecular Crystals
Organic molecular crystals frequently exist in multiple forms known as polymorphs. Structural differences between crystal polymorphs can affect desired properties, such as bioavailability of active pharmaceutical formulations, lethality of pesticides, or electrical conductivity of organic semiconductors. Crystallization conditions can influence polymorph selection, making an experimentally driven hunt for polymorphs difficult. Such efforts are further complicated when polymorphs initially obtained under a particular experimental protocol “disappear” in favor of another polymorph in subsequent repetitions of the experiment. Consequently, theory and computational can potentially play a vital role in mapping the landscape of crystal polymorphism. However, traditional theoretical methods face their own challenges, and new approaches are needed. In this talk, I will show, by leveraging concepts from statistical mechanics in combination with techniques of molecular simulation and machine learning, a that new paradigm in crystal structure prediction may be emerging.
Andy Teale
School of Chemistry, University of Nottingham, UK
All electron approaches to orbital-free density-functional theory
Andy Teale, Matt Ryley and Tom Irons: School of Chemistry, University of Nottingham, UK
Trygve Helgaker: Hylleraas Centre for Quantum Molecular Sciences, Department of Chemistry, University of Oslo, Norway
The development of an accurate orbital-free approach to density-functional theory for general finite and periodic systems has long been an elusive goal for quantum chemists. Much progress has been made for periodic systems with local pseudopotentials, e.g. PROFESS [1] and ATLAS [2]. However, robust all electron treatments have lagged behind these developments. Recently, Lopez-Acevedo [3, 4] has shown how an all electron implementation (GPAW) of OF-DFT calculations can be achieved for the Thomas-Fermi von Weisacker family (ɣTFλW) of kinetic energy density functionals (KEDFs). This approach uses ideas based on work by Levy, Perdew and Sahni [5], who identified the equivalence between the solution of the Euler equation and a one-orbital analogue of the Kohn-Sham equations. To achieve convergence using the approach of Ref. [3] it is necessary to employ simple Pulay-type density matrix damping, rather than conventional SCF accelerators such as Pulay’s C1-DIIS; leading to a large number of iterations, see Fig. 1. In addition, as discussed by Karasiev and Trickey [6], extension of this approach to other KEDFs is unclear. Furthermore, differing conclusions as to the stability of different KEDFs have been reached using different programs.
Earlier Chan, Cohen and Handy [7] proposed an all electron approach for finite systems using Gaussian functions, which is applicable to general KEDFs. In their initial publication only applications to ɣTFλW functionals were presented. This approach uses a nested optimization of the OF-DFT Langrangian to determine the ground state energy and chemical potential μ. Here we present a new implementation of this scheme [8], which can be applied to a wide range of KEDFs using a flexible framework based on automatic differentiation techniques afforded by the XCFun library [9]. This approach provides a reasonably robust all electron approach to optimization using a variety of KEDFs. However, the nested nature of the optimization can lead to many steps to be required for convergence, see Fig 2.
To address this issue, we have developed a new trust-region image (TRIM) based optimization method for OF-DFT [10]. This second order approach simultaneously optimizes E and μ, leading to a significant reduction in the number of steps required for convergence and very precise quadratic convergence in the local region. Prospects for future use and development of this approach will be discussed. Presently, this approach provides a platform testing KEDFs in a fully self-consistent all electron context. Results demonstrating the essential requirement to consider self-consistency when designing new KEDFs will be discussed.
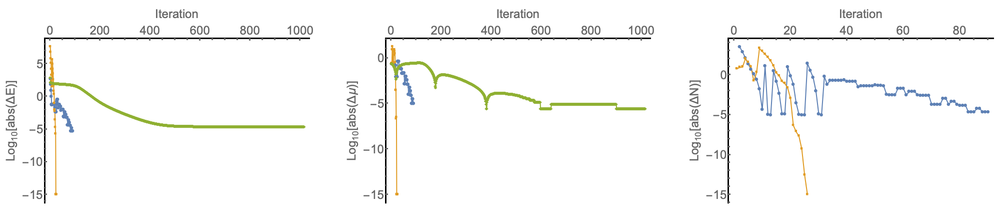
Figure 1: Ne atom, with ɣTFλW (ɣ = 0.697, λ = 0.599) and Dirac exchange. Convergence profiles for the Lopez-Acevedo approach (green), the Chan, Cohen, Handy method (blue), and the TRIM approach (orange). The change in the electronic energy, chemical potential and the particle number error are shown as a function of the number of iterations. Note that for the particle number variation the Lopez-Acevedo approach is normalized to the correct number of particles at each iteration by construction and the chemical potential in this scheme is determined by the lowest eigenvalue of the effective Hamiltonian.
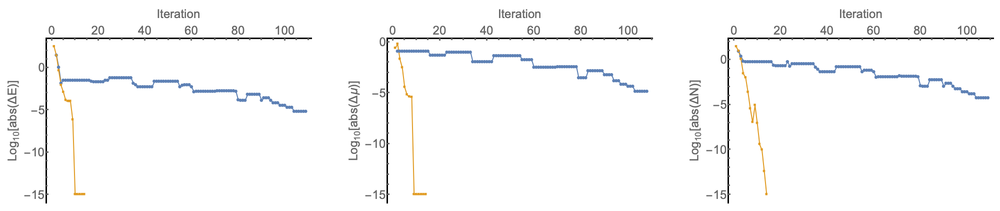
Figure 2: N2 molecule with the E00 KEDF and PBE exchange and correlation. Convergence profiles for the Chan, Cohen, Handy method (blue), and the TRIM approach (orange). A superposition of atomic densities guess is used, calculated with the same functionals. The change in the electronic energy, chemical potential and the particle number error are shown as a function of the number of iterations.
References
[1] G. S. Ho, V. L. Lignères, and E. A. Carter, Comput. Phys. Commun. 179, 839 (2008)
[2] W. Mi, X. Shao, C. Su, Y. Zhou, S. Zhang, Q. Li, H. Wang, L. Zhang, M. Miao, Y. Wang, Y. Ma, Comput. Phys. Commun. 200, 87 (2016)
[3] J. Lehtomäki, I. Makkonen, M. A. Caro, A. Harju and O. Lopez-Acevedo, J. Chem. Phys. 141, 234102 (2014)
[4] L. A. Espinosa Leal, A. Karpenko, M. A. Caro and O. Lopez-Acevedo, Phys. Chem. Chem. Phys. 17, 31463 (2015)
[5] M. Levy, J. P. Perdew and V. Sahni, Phys. Rev. A 30, 2745 (1984)
[6] V. Karasiev and S. Trickey, Comput. Phys. Commun. 183, 2519 (2012)
[7] G. K.-L. Chan, A. J. Cohen and N. C. Handy, J. Chem. Phys. 114, 631 (2001)
[8] QUEST, a rapid development platform for QUantum Electronic Structure Techniques, http://quest.codes
[9] U. Ekström, L. Visscher, R. Bast, A. J. Thorvaldsen and K. Ruud, J. Chem. Theory Comput. 6, 1971 (2010)
[10] T. Helgaker, Chem. Phys. Lett. 182, 5, 503 (1991)
Marcos Mandado
Department of Physical Chemistry. Faculty of Chemistry. University of Vigo. 36310 Vigo. Galicia. Spain
Detecting plasmons at molecular scale by means of detachment/attachment electron density analysis
Marcos Mandado, Sara Gil-Guerrero, Ángeles Peña-Gallego: Department of Physical Chemistry. Faculty of Chemistry. University of Vigo. 36310 Vigo. Galicia. Spain
Collective electronic excitations in extended surfaces, also called plasmons, are represented by electron density oscillations using classical electromagnetic theory, nevertheless, a quantum definition for extended systems is obtained from linear response theory. Recent theoretical and experimental works have pointed out the presence of plasmon excitations at molecular scale as well, for instance, in metallic clusters, fullerenes or small and medium size nanotubes.[1-4] However, the definition of plasmon at molecular scale cannot be directly connected to that in extended systems as the key concept, the dielectric function, has not a straightforward analogy in molecules or clusters. Recently, using solid theoretical arguments, Bernadotte et al [1] have shown that plasmon character in finite systems can be attached to excitation modes strongly dependent on the response of the electron-electron operator, whereas those almost independent correspond to single-electron excitations. However, differentiation between single-electron and collective modes requires the introduction of an arbitrary multiplicative parameter in the electron-electron part of the Casida equation, which is varied gradually from 1 to 0 to connect the correct solutions with those obtained by the noninteracting electron picture. Unfortunately, this procedure entails a large number of calculations and is tricky when different excitation modes cross or collapse at an intermediate value of the parameter.
In this presentation, it is shown how an alternative differentiation between plasmons and single-electron excitations can be done at molecular scale by means of the attachment/detachment integrated density obtained from time-dependent linear response theory. This integrated density represents the electron population excited from the occupied to the virtual orbital band. Those excitations whose integrated density is close to the ideal value 1.0 correspond to single-electron excitations, whereas those whose integrated density is significantly higher than 1.0 are characterized as collective excitations or plasmons.[5] By the way, high values of the integrated density are linked to large weights of deexcitation terms, which are well-known to be key to representing the electron density oscillations associated to plasmon modes.[6]
References
[1] S. Bernadotte, F. Evers, C. R. Jacob, J. Phys. Chem. C 117, 1863, (2013)
[2] A. Crai, A. Pusch, D. E. Reiter, L. Román Castellanos, T. Kuhn, O. Hess, Phys. Rev. B 98, 165411, (2018)
[3] A. Manjavacas, F. Marchesin, S. Thongrattanasiri, P. Koval, P. Nordlander, D. Sánchez-Portal, F. J. García de Abajo, ACSNano 7, 3635, (2013)
[4] A. Lauchner, A. E. Schlather, A. Manjavacas, Y. Cui, M. J. McClain, G. J. Stec, F. J. García de Abajo, P. Nordlander, N. J. Halas, Nano Lett. 15, 6208, (2015)
[5] S. Gil-Guerrero, A. Peña-Gallego, M. Mandado, in preparation
[6] M. Grüning, A. Marini, X. Gonze, Nano Lett. 9, 2820, (2009)
Verònica Postils
Departamento de Ciencia y Tecnología de Polímeros, Kimika Fakultatea, Euskal Herriko Unibertsitatea (EHU). Paseo Manuel de Lardizabal 3, 20018 Donostia (Gipuzkoa, Spain)
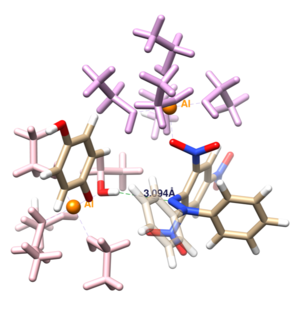
Pro-oxidant activity of aluminum promoting radical-scavenging reactions. DPPH-QH2 pair as a test case.
José Manuel Lanuza, Xabi López and Verònica Postils
Aluminum is known to play an important role in biological systems altering the thermodynamics of a variety of proteins and biomolecules (e.g. specially those containing phosphate and carboxylate groups)[1] and affecting key redox reactions such the Fenton reaction of Fe3+/Fe2+ among others.[2] The formation of an Al-superoxide (O2·-) complex has been hypothesized to be central for this oxidant activity.[3]
Recently, experimental evidences have exhibited that the presence of aluminum promotes the scavenging reaction of dihydroquinone (QH2) with 2,2-diphenyl-1-picrylhydrazyl radical (DPPH·),[4] (Equation 1) being those reagents frequently used to model the reactivity between the antioxidant co-enzyme Q10 and reactive oxygen(nitrogen) species (ROS), respectively. The study concludes that Al3+, as a strong Lewis acid, acts as a radical scavenging promoter because it stabilized more the one-electron reduced species of DPPH· (i.e. DPPH-) than acts as a pro-oxidant, inhibiting the antioxidant activity of QH2.
We have performed a DFT computational study for the scavenging reaction of QH2 with DPPH·, both with and without the presence of Al3+. We have computed their effective redox potentials and identified the necessary conditions for the reaction to occur. Our computational study also reveals how the stabilization of negatively charged anionic reactive oxygen/nitrogen species (i.e. the stabilization of DPPH-) is a key aspect in the influence of Al3+ in the scavenging reaction, finding a good agreement between theory and experiments. Moreover, the study provides new insights about intermediate species involved in the redox reaction and the reaction mechanism, which may help to understand Al effects in the activity of the antioxidant co-enzyme Q10.
References
[1] a) Luque, N. B.; Mujika, J. I.; Rezabal, E.; Ugalde, J. M.; López, X. Phys. Chem. Chem. Phys. 2014, 16, 20107-20119. (b) Mujika, J. I.; Ugalde, J. M.; López, X. J. Phys. Chem. B. 2014, 118, 6680-6686. [2] Ruipérez, F.; Mujika, J. I.; Ugalde, J. M.; Exley, C.; López, X. J. Inorg. Biochem. 2012, 117, 118-123. [3] Exley, C. Free Radic. Biol. Med. 2004, 36, 380-387. [4] Nakanishi, I.; Ohkubo, K.; Ogawa, Y.; Matsumoto, K.; Ozawa, T.; Fukuzumi, S. Org. Biomol. Chem. 2016, 14, 7956-7961.
Henryk Witek
Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung University, Hsinchu 30010, Taiwan
Problems with multireference perturbation theory
Quantum chemical calculations of physical and chemical properties of molecular systems in their excited states are often performed using multireference perturbation theory (MRPT). MRPT comes in many flavors and is implemented in many various quantum chemistry packages. In the current talk I would like to review a number of serious methodological problems in MRPT, which make the method in many instances difficult or even impossible to apply for a non-specialist. I hope that the thorough discussion of the discovered problems might be of help for anybody planning to compute potential energy surfaces for excited states.
References
1. Y.-K. Choe, H. A. Witek, J. P. Finley, and K. Hirao, “Identifying and Removing Intruder States in Multireference Møller-Plesset Perturbation Theory”, J. Chem. Phys. 114, 3913 (2001)
2. H. A. Witek, Y.-K. Choe, J. P. Finley, and K. Hirao, “Intruder State Avoidance Møller-Plesset Perturbation Theory”, J. Comput. Chem. 23, 957 (2002)
3. H. A. Witek, N. Nakano, and K. Hirao, “Multireference Perturbation Theory with Optimized Partitioning. I. Theoretical and Numerical Aspects”, J. Chem. Phys. 118, 8197 (2003)
4. H. A. Witek, N. Nakano, and K. Hirao, “Multireference Perturbation Theory with Optimized Partitioning. II. Application to Molecular Systems”, J. Comput. Chem. 24, 1390 (2003)
5. C. Camacho, S. Yamamoto, and H. A. Witek, “Choosing a proper complete active space in calculations for transition metal dimers: Ground state of Mn2 revisited”, Phys. Chem. Chem. Phys. 10, 5128 (2008)
6. C. Camacho, S. Yamamoto, and H. A. Witek, “Intruder states in multireference perturbation theory: ground state of Mn2”, J. Comput. Chem. 30, 468–478 (2009)
7. C. Camacho, R. Cimiraglia, and H. A. Witek, “Multireference perturbation theory can predict a false ground state”, Phys. Chem. Chem. Phys. 12, 5058–5060 (2010)
8. C. Camacho, H. A. Witek, and R. Cimiraglia, “The low-lying states of the scandium dimer”, J. Chem. Phys. 132, 244306/1–9 (2010)
9. M. Andrzejak and H. A. Witek, “The elusive excited states of bithiophene: A detective CASPT2 story”, Theor. Chem. Acc. 129, 161–172 (2011)
10. S. W. Chang and H. A. Witek, “Choice of optimal shift parameter for the intruder state removal techniques in multireference perturbation theory”, J. Chem. Theory Comp. 8, 4053–4061 (2012)
11. M. Andrzejak, M. Kukułka, H.A. Witek, “Excited states manifold of 2,2’-bithiophene: basis set dependence study”, Mol. Phys. 115, 2823–2832 (2017)
Raúl Quintero-Monsebaiz
Alberto Vela and Raúl Quintero-Monsebaiz: Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV).México
Ion Mitxelena, Mauricio Mayorga and Mario Piris: Donostia International Physics Center (DIPC).España
Spin Polarized Natural Orbital Functional Theory
Within Natural Orbital Functional Theory, a spin-uncompensated formulation for the independent pair approach PNOF5[1][2] and the interacting pair model PNOF7[3][4] was proposed. Each one of these functionals fulfills certain N-representability necessary conditions of second-order reduced density matrices. The spin symmetry is also accomplished taking into account the conservation of total spin. A benchmarking was made using the one dimensional Hubbard model with periodic boundary conditions[5]. The spin-polarized functionals show the correct description of strong-correlation effects.
References
[1] M. Piris, X. Lopez, F. Ruipérez, J. M. Matxain, and J. M. Ugalde, J. Chem. Phys. 134, 164102 (2011).
[2] M. Piris, J. M. Matxain, and X. Lopez, J. Chem. Phys. 139, 234109 (2013).
[3] M. Piris, Phys. Rev. Lett. 119, 063002 (2017).
[4] I. Mitxelena, M. Rodríguez-Mayorga, and M. Piris, Eur. Phys. J. B91, 109 (2018).
[5] R Quintero, I. Mixtelena,M. Rodriguez,A. Vela, M. Piris, J. Phys: Condens Matter, (2019).
Eloy Ramos-Cordoba
Donostia International Physics Center (DIPC), Donostia, Euskadi, Spain
Singling out Strong and Weak correlation
The correlation part of the pair density is separated into two components, one of them being predominant at short electronic ranges and the other at long ranges. The analysis of the intracular part of these components permits to classify molecular systems according to the prevailing correlation: dynamic or nondynamic. The study of the long-range asymptotics reveals the key component of the pair density that is responsible for the description of London dispersion forces and a universal decay with the interelectronic distance. The natural range-separation, the identification of the dispersion forces and the kind of predominant correlation type that arise from this analysis are expected to be important assets in the development of new electronic structure methods in wavefunction, density, and reduced density-matrix functional theories. In particular, within the framework of Kohn-Sham density functional theory, the partition reveals a simple structure for the two pair density components.
Alberto Vela
Luis Soriano; Departamento de Química; Centro de Investigación y de Estudios Avanzados (Cinvestav-Zacatenco) Av. Instituto Politécnico Nacional 2508, 07360 Ciudad de México, México
Can we predict spin crossover in transition metal complexes?
Spin-crossover (SCO) in transition metal complexes is of great importance in the development of magnetic materials whose properties are used in visualization, memory and electrical devices, to mention a few. This phenomenon has been widely studied with Density Functional Theory (DFT) using a wide variety of exchange-correlation (XC) functionals with disappointing outcomes, pointing towards the incapability of current Density Functional Approximations (DFAs) in reliably predicting the energy differences between the high- and low-spin configurations in transition metal complexes. Recently, an approach based on density corrected DFT and called HF-DFT, consisting on evaluating the energy of a selected DFT functional with the Hartree-Fock (HF) density has been successfully applied to several problems, including SCO in some iron complexes with small ligands, yielding results that are close to coupled cluster (CC) and Diffusion Monte Carlo calculations, at a much lower computational cost. In this work we show that HF-DFT, using DFT optimized geometries, also offers an excellent alternative to describe SCO in manganocenes with ligands in the cyclopentadienyl rings going from hydrogen to tert-butyl, and again, providing results in excellent agreement with available CCSD calculations. By means of multiconfigurational-SCF calculations we show that these systems have an important contribution of static correlation. We also show that it is almost impossible to obtain gas-phase energy differences between the high and low spin configurations that can allow one a reliable prediction of SCO. In the case of neutral complexes, like manganocenes, the only approach that we have found to be robustly predictive for the existence of SCO is the periodic calculation, using the geometry of the complexes in the solid state. For charged complexes, like those containing iron, including the counterions seems to distort enough the crystal field of the transition metal, leading to high-low spin energy differences in agreement with the observation of SCO.
Mercedes Alonso
General Chemistry Department (ALGC), Vrije Universiteit Brussel (VUB), Pleinlaan 2, Brussels, Belgium
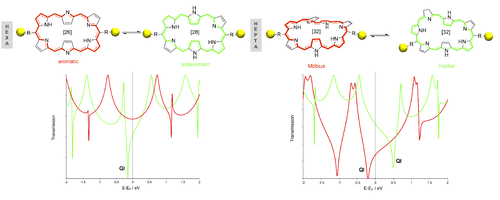
Towards the design of molecular conductance and bithermoelectric switches
Creating functional nanoscale devices using single molecules as active electronic components is the ultimate goal of the field of molecular electronics. Besides their potential to meet the growing demand for miniaturization of electronics, molecular electronics opens up the possibility of devices with novel, unforeseen functionalities beyond silicon-based technologies, such as molecular switches. Through a bottom-up quantum chemistry approach, we have shown that expanded porphyrins are flexible enough to switch between different π-conjugation topologies (Mobius, Hückel and twisted-Hückel) encoding distinct electronic properties and aromaticity.[1] Since these topology/aromaticity switches can be induced by different external stimuli,[2] these macrocycles represent a unique platform to develop a novel type of molecular switches for different nanoelectronic applications.
The first application involves the conductance switching in molecular junctions through aromaticity and topology changes. In this regard, the electron transport properties of the different states of the switches were carefully investigated with the non-equilibrium Green´s function formalism in combination with density functional theory for various configurations of the gold contacts.[3] Our findings reveal that the negative relationship between conductance and molecular aromaticity or polarizability does not hold for most of the configurations of the molecular junctions, so we devise new selection rules to predict the occurrence of quantum interference around the Fermi level for Hückel and Möbius systems.[4] A second application concerns the design of bithermoelectric switches, an entirely new class of switches that revert the direction of the heat and/or charge transport. Our in-house calculations reveal that the Hückel-Möbius switch in heptaphyrins causes the Seedbeck coefficient or thermopower to change considerably from +50 μV/K to -40 μV/K.[5] Chemical control over the thermoelectric properties can be achieved through substitution of the linker groups. Overall, our work demonstrates how the concept of aromaticity and molecular topology can be exploited to create efficient switching devices.
References
[1] M. Alonso, P. Geerlings, F. De Proft, Chem. Eur. J. 2013, 19, 1617.
[2] M. Alonso, B. Pinter, P. Geerlings, F. De Proft, Chem. Eur. J 2015, 21, 17631; T. Woller, J. Contreras-García, P. Geerlings, F. De Proft, M. Alonso, Phys. Chem. Chem. Phys. 2016, 18, 11885.
[3] T. Stuyver, F. De Proft, P. Geerlings, M. Perrin, M. Alonso, J. Am. Chem. Soc. 2018, 140, 1313.
[4] T. Stuyver, S. Fias, P. Geerlings, F. De Proft, M. Alonso, J. Phys. Chem. C 2018, 122, 19482.
[5] T. Stuyver, P. Geerlings, F. De Proft, M. Alonso, J. Phys. Chem. C 2018, 122, 24436.
Carla Calvó-Tusell
Institute of Computational Chemistry and Catalysis and Department of Chemistry, University of Girona, E-17003 Girona, Catalonia, Spain.
Unraveling the millisecond allosteric activation of Imidazole Glycerol Phosphate Synthase (IGPS) by means of Molecular Dynamics simulations
Carla Calvó-Tusell, Miguel A. Maria-Solano, Ferran Feixas, Sílvia Osuna
Allostery is an intrinsic property of proteins generally described as the process by which the effect of binding at one site is transmitted to another, often distal, functional site, allowing for activity or function regulation.[1] Exploring the mechanisms of allosteric regulation is important to understand biological processes such as enzyme catalysis, cell signaling or the molecular basis of disease. Allosteric transitions require the order of microsecond to second timescales. Imidazole Glycerol Phosphate Synthase (IGPS) is an heterodimeric enzyme complex widely used as a model to study allosteric regulation between subunits. Previous studies based on NMR experiments reported that the allosteric activation of IGPS, promoted by the binding of an effector, takes place in the millisecond timescale.[2] However, the active structure of IGPS has not been yet characterized.[2,3] Computational methods are used to study allostery, but obtaining enough conformational sampling to completely characterize allosteric events represents a challenge for current simulation techniques.[4]
In this work, the millisecond allosteric activation mechanism of IGPS is unraveled step by step by means of a combination of simulation techniques. First, nanosecond to microsecond motions that initiate the allosteric signal transmission are deciphered by means of conventional Molecular Dynamics (cMD). Second, accelerated Molecular Dynamics (aMD) is used to sample events that occur in the microsecond to millisecond timescale, including the complete characterization of IGPS allosteric activation and the active structure (characterized by the formation of an oxyanion hole). Third, the complete reconstruction of substrate binding process is performed to characterize the reactive complex of the allosterically activated enzyme. Then, the reaction mechanism of inactive and allosterically active IGPS are studied by means of density functional theory calculations. Finally, the allosteric communication pathway is traced through key residues for IGPS function and allostery using correlation-based tools.[5] In summary, we have been able to computationally characterize the activation mechanism of IGPS by applying a computational protocol designed to describe and characterize complex long timescale motions. This protocol can be generalized to study the activation mechanism of other allosterically regulated enzymes.
References:
[1] VanWart, A. T.; Eargle, J.; Luthey-Schulten, Z.; Amaro, R. E., J.Chem Theory Comput. 2012, 8, 2949-2961
[2] Lipchock, J. M. Loria, J. P., Structure, 2010, 18, 1596-1607
[3] Rivalta, I., Sultan, M. M.; Lee, N. S.; Manley, G. A.; Loria, J. P.; Batista, V. S., Proc. Natl. Acad. Sci. U.S.A., 2012, 109, 1428-1436
[4] Wagner, J. R., Lee, C. T., Durrant, J. D., Malmstrom, R. D., Feher, V. A., Amaro, R. E., Chem. Rev., 2016, 116, 11, 6370-6390
[5] Romero-Rivera, A.; Garcia-Borràs, M.; Osuna, S., ACS Catalysis, 2017, 3, 949-960
Ferran Feixas
Institut de Química Computacional i Catàlisi (IQCC) and Departament de Química, Universitat de Girona, c/ Maria Aurèlia Capmany 69, 17003, Girona, Catalonia, Spain
Understanding the role of non-covalent interactions in (bio)molecular recognition
(Bio)molecular recognition and assembly are key concepts in chemistry, biology and drug design. Chemical and life processes are critically dependent on the association and dissociation of (bio)molecules. A deep understanding of the mechanisms of these relevant processes is of utmost importance. Experimental methods can provide information on stable structures, binding affinities, or kinetics but cannot offer a complete description of binding and unbinding pathways at the required atomic detail. Molecular dynamics can provide an atomistic dynamic view of such relevant processes. However, a tremendous amount of conformational sampling is required to obtain accurate pathways and reliable stationary and kinetic properties. Current computational methods to study assembly, binding, and unbinding of (bio)molecules either require access to high-performance computing resources or the definition of a complex reaction coordinates to enhance conformational sampling.
Our goal is to develop a computational protocol to study, at the atomic level, the mechanisms of self-assembly, association and dissociation of (bio)molecules at a reasonable computational cost. This protocol relies on the basic ideas of accelerated molecular dynamics (aMD),[1,2] an unconstrained enhanced sampling technique that does not rely on the a priori definition of any reaction coordinate. The novel strategy consists in the redefinition of this methodology by selectively boosting non-bonded interactions between interacting molecules to enhance conformational sampling of associative and dissociative processes. This protocol is assessed for well-studied processes including protein folding, biomolecular recognition and protein-protein interactions. In particular, we focus on the potential of this novel technique as a tool to efficiently explore the rough free energy landscape of binding and unbinding in proteins and supramolecular systems. First, we focus on understanding enzyme-inhibitor binding process in a p38 protein kinase liver cancer drug target.[3] From MD simulations, we can reconstruct the free energy landscape of the binding process and identify relevant states of this process. Using, tools such as the non-covalent interaction index (NCI) and electron sharing indices we can rationalize the stability of the interactions established and improve the activity of the inhibitor. Second, we explore the dynamic role of non-covalent interactions in a series of host-guest systems including self-folding cavitands and supramolecular cages specifically designed for binding and release of fullerenes.
References
[1] Markwick, P. R. L.; McCammon, J. A. Phys. Chem. Chem. Phys. 2011, 13, 20053
[2] Miao, Y.; Feixas, F.; Eun, C.; McCammon, J. A. J. Comput. Chem. 2015, 36, 1536
[3] Tomás-Loba, A.; Manieri, E.; González-Terán, B.; Mora, A.; Leiva-Vega, L.; Santamans, A. M.; Rodríguez, E.; Feixas, F.; López, J. A.; Caballero, B.; Trakala, M.; Blanco, O.; Torres, J. L.; Roche-Molina, M.; Bernal, J. A.; Saavedra, D. M.; Ruiz-Cabello, J.; Brava-Sicilia, J.; Vázquez, J.; Marcos, M.; Malumbres, M.; Osuna, S.; Sabio, G. Nature 2019, 568, 557
José L. Gázquez
Héctor Francisco-Rodríguez and Javier Carmona-Espíndola - Departamento de Química, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco 186, Ciudad de México, 09340, México.
Analysis of the kinetic energy functional in the generalized gradient approximation
The kinetic energy in the generalized gradient approximation (GGA) is expressed as the product of the local density approximation and an enhancement factor that is a function of the reduced density gradient, s. The expression for the enhancement factor F(s) that characterizes this approximation, can be built to fulfill different constraints that the exact functional satisfies. In the present work we perform first a comparison between the aspects that characterize the GGA approach for the exchange and kinetic energies, to conclude that the conjoint gradient correction may be considered as a criterion to establish the general form of F(s). However, it is important to incorporate into this general form the correct small s and large s limits that correspond to the kinetic energy. For the latter, we make use of non-uniform coordinate scaling to show that the kinetic energy enhancement factor must diverge as s square. Then we make an interpolation between the two limits based, partially, in the mathematical expression of the PBE exchange enhancement factor, to derive a non-empirical kinetic energy functional that leads to a reasonable description of kinetic energies, when these are determined with Hartree-Fock densities.
Rika Kobayashi
ANU Supercomputer Facility, Leonard Huxley Bldg 56, Mills Rd, Canberra, ACT, 2601, AUSTRALIA
Machine Learning in Chemistry and Materials Science
In recent years technological advances in high-performance computing has focused on architectures based on high-density chips suitable for accelerated computing. The high compute – low data handling capability of GPUs has made people realise how ideal GPU chips are for machine learning thus encouraging many computational chemists and materials scientists to investigate potential application to their field. I will give a brief introduction to the principles of Machine Learning and show some examples of how it can be applied to chemistry and materials science.
References
[1] S. T. Hutchinson and R. Kobayashi, Solvent-Specific Featurisation for Predicting Free Energies of Solvation through Machine Learning, J. Chem. Inf. and Model. (DOI: 10.1021/acs.jcim.8b00901)
[2] R. D. Amos and R. Kobayashi, Feature Engineering for Materials Chemistry - Does Size Matter?, J. Chem. Inf. and Model. (DOI: acs.jcim.8b00977)
Vincent Ortiz
Department of Chemistry and Biochemistry, Auburn University, Auburn AL 36849-5312 U.S.A.
Rydberg Anions, Solvated-Electron Precursors and Their Dyson Orbitals
Highly correlated, diffuse electron pairs that surround saturated, closed-shell cations are present in species such NH4- and OH3- that are known as double Rydberg anions. Dyson orbitals, calculated with renormalized ab initio electron-propagator methods for electron detachment energies, are distributed chiefly outside the cationic core, have nonbonding character and resemble the 3s united-atom limit of Na-. Addition of ammonia molecules to NH4- results in formation of multiple NnH3n+1- isomers with distinct delocalization patterns in their Dyson orbitals of electron detachment, especially when cationic cores have hydrogen bridges. In solvated-electron precursors (SEPs), dative bonding from ammonia lone pairs to diamagnetic or paramagnetic metal di-cations is strong enough to displace valence s electrons of metal atoms to the periphery of these M(NH3)n complexes . Dyson orbitals for ionization energies of SEPs are markedly more diffuse than metal valence s functions and constructive interference between them results in formation of SEP dimers and clusters. Electron-propagator calculations of electron attachment energies and their Dyson orbitals reveal an Aufbau principle that is applicable to a variety of species with diffuse electrons.
Ria Broer
Zernike Institute for Advanced Materials, University of Groningen, The Netherlands, email: r.broer@rug.nl
Theoretical Chemistry for the Design of Functional Materials
Theoretical and computational chemistry play increasingly important roles in chemistry and physics, thanks to clever methods and codes, combined with powerful compute facilities that allow for accurate simulation of chemical and physical processes. Calculations can replace and complement experiments when needed and provide essential information, for example for designing new (functional) materials. Another important task of theoretical chemistry is to elucidate the mechanisms of chemical and physical processes by careful and systematic analysis of computed results.
In this presentation two examples will be discussed that are relevant for the field of organic photovoltaics. The first is a recent quantum chemical investigation of the factors that determine whether a compound shows singlet fission, a process that leads to the generation of two excitons after absorption of one photon [1]. The second example concerns the development and application of a multiscale computational scheme to calculate the electronic and nuclear contributions to the dielectric constant of organic semiconductors [2]. Our results unravel the timescale of these contributions and shed light on their relevance for high efficiency photovoltaics.
A last example is the study of spin crossover processes, which may occur in metal-organic complexes of d4 ... d7 transition metal ions. So-called spin crossover complexes have two low-energy states, one with maximum electron pairing and one with a maximum number of unpaired d electrons. A transition or “crossover” between these states can lead to “excited spin-state trapping”, which is of technological importance. The crossover mechanism may involve various intersystem crossings and internal conversions and can be unraveled by combining quantum chemical calculations with a time-dependent formalism for calculating intersystem crossing rates [3].
References [1] M. Wibowo, R. Broer, and R.W.A. Havenith, Comput. Theor. Chem. 1116, 190 (2017). [2] S. Sami, P.A.B. Haase, R. Alessandri, R. Broer, and R.W.A. Havenith, J. Phys. Chem. A 122, 3919 (2018). [3] A. Rudavskyi, C. Sousa, C. de Graaf, R.W.A. Havenith, and R. Broer, J. Chem. Phys. 140, 184318 (2014); C. Sousa, C. de Graaf, A. Rudavskyi, and R. Broer, J. Phys. Chem. A 121, 9720 (2017).
Roi Baer
Fritz Haber Center for Molecular Dynamics, Institute of Chemistry, the Hebrew University of Jerusalem
Unraveling nonequilibrium dynamics of non-interacting electrons in open systems
The Lindblad equation describes quantum dynamics in systems that cannot be completely isolated from an environment, addressing dissipation and decoherence phenomena. Numerous applications of the formalism exist for small Hilbert-space systems, such as an atom or a molecule. But for many-electron systems, the dynamics are intractable even if Coulomb repulsion could be switched off since the particles would still be able to affect each other by interacting with the bath. Here, we develop an approximate approach for evolving non-interacting Fermions in open quantum systems based on the following elements [1]:
1) The form of frequency-dependent Lindblad operators as formulated by Davies [2],
2) The unraveling procedure for converting the Lindblad equation into a stochastic Schrodinger equation [3].
3) Construction of time-dependent stochastic Lindblad operators.
4) Deploying the Hubbard-Stratonovich transformations [4-5] to represent the two-body interactions.
5) A collapsing procedure between time steps.
The method aims for open quantum systems that include thousands of electrons and dense bands. Achieving this goal is wanting.
References
[1] Ruan, Z. and Baer, R. Unravelling open-system quantum dynamics of non-interacting Fermions. Mol. Phys. 116, 2490-2496 (2018).
[2] Davies, E. B., Markovian master equations, Communications in Mathematical Physics, 39, 91-110 (1974).
[3] Gisin, N., and Percival, I. C. The Quantum-State Diffusion-Model Applied to Open Systems, Journal of Physics a-Mathematical and General, 25, 5677–5691, (1992).
[4] Hubbard J. Calculation of Partition Functions, Phys. Rev. Lett., 3, 77 (1959).
[5] Stratonovich, R. L. A method for the computation of quantum distribution functions, Dokl. Akad. Nauk SSSR, 115, 1097 (1957).
John P. Perdew
Department of Physics, Temple University, Philadelphia, PA 19122, USA
Strong constraints and appropriate norms in density functional theory
Approximations to the density functional for the exchange-correlation energy of a many-electron system require exact constraints (mathematical properties of the exact functional) and appropriate norms (systems for which the approximation can be exact or nearly exact). The SCAN meta-generalized gradient approximation [1] has carried this strategy further than other approximations, with remarkable success. SCAN satisfies 17 exact constraints, and augments the uniform-gas appropriate norms by non-bonded systems such as atoms. SCAN recognizes and describes diverse bonds, including covalent, metallic, and van der Waals [2]. It correctly describes small energy differences in ferroelectrics [3] and water [4], the formation energies and ground-state crystal structures of many solids [5], and the critical pressures of structural phase transitions [6]. Possibilities for further improvement beyond SCAN will also be discussed.
References [1] J. Sun, A. Ruzsinszky, and J.P. Perdew, Phys. Rev. Lett. 115, 036402 (2015). [2] J. Sun, R.C. Remsing, Y. Zhang, Z. Sun, A. Ruzsinszky, H. Peng, Z. Yang, A. Paul, U. Waghmare, X. Wu, M.L. Klein, and J.P. Perdew, Nature Chem. 8, 831 (2016). [3] Y. Zheng, J. Sun, J.P. Perdew, and X. Wu, Phys. Rev. B 96, 035143 (2017). [4] M. Chen, H.-Y. Ko, R.C. Remsing, M.F. Calegari Andrade, B. Santra, Z. Sun, A. Selloni, R. Car, M.L. Klein, J.P. Perdew, and X. Wu, Proc. Nat. Acad. Sci. USA 114, 10846 (2017). [5] Y. Zhang, D.A. Kitchaev, J. Yang, T. Chen, S.T. Dacek, R.A. Sarmiento-Perez, M.A.L. Marques, H. Peng, G. Ceder, J.P. Perdew, and J. Sun, NPJ Computational Materials 4, 9 (2018). [6] C. Shahi, J. Sun, and J.P. Perdew, Phys. Rev. B 97, 094111 (2018).
Peter M.W. Gill
Research School of Chemistry, Australian National University, Canberra, Australia
Q-MP2-OS: A new approach to correlation using quadrature
As computational hardware becomes ever more massively parallel, quantum chemical methods and their underpinning implementations must evolve. In this lecture, I will present a novel algorithm for the computation of the opposite-spin (OS) MP2 correlation energy, which is well suited to large-scale parallelization. The method combines deterministic numerical quadratures and screening techniques, and entirely bypasses the computation of two-electron integrals. Speedup, scaling and accuracy results for a variety of molecules and reactions reveal that the new algorithm achieves 1 kcal/mol accuracy with almost perfect parallelizability and a computational cost which grows only quadratically with system size. [1]
References
[1] G.M.J. Barca, S.C. McKenzie, N.J. Bloomfield, A.T.B. Gilbert and P.M.W. Gill, submitted.
Pedro Salvador
Institut de Química Computacional i Catàlisi, Universitat de Girona, Spain
Energy-based origin-independent decomposition of linear and nonlinear optical properties
Historically, there has been great interest in decomposing the global value of linear and nonlinear optical properties (NLOP) into contributions of individual atoms or functional groups. For instance, transferable group polarizabilities would give insight about the value of the NLOP, and also allow for their prediction, making it possible to rationally design particular molecular systems with high NLOP. The main problem in this strategy was the lack of origin-independent decompositions of NLOP, meaning that the contribution of atoms or functional groups depend not only on their nature, but also on their position with respect to the origin of coordinates. Throughout the years this topic has been studied, quantifying the limitations of the origin-dependence,[1] and also suggesting some connectivity-dependent alternatives that try to circumvent the problem.[2]
Most recently,[3] we have found a general energy-based decomposition or NLOP into atomic or function group contributions that are genuinely origin-independent. We will show how they can be derived and will provide proper comparision to alternative approaches to compute group polarizability contributions. If time permits, we will also discuss our most recent findings in the context of Kohn-Sham molecular energy decompostion schemes, in which the decomposition of the optical properties are rooted.
References
[1] Y. Mei, A. C. Simmonett, F. C. Pickard, IV, R. A. DiStasio, Jr., B. R. Brooks, and Y. Shao J. Phys. Chem. 2015, 119, 5865-5882. [2] T. A. Keith, Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007. (pp. 61-94) [3] M. Montilla, J.M. Luis and P. Salvador, in preparation.
Vladimiro Mujica
Arizona State University, School of Molecular Sciences Tempe, Arizona 85287-1604
Phonon Filters in Molecular Junctions
We present here an extensive computational study demonstrating that heterogeneous molecular junctions, consisting of molecular wires bridging two different nanocontacts, can act as a selective phonon filter. This filtering effect leads in turn to asymmetric energy transfer in the junctions, which is a fundamental problem for thermal energy harvesting in molecular devices. The most important finding is the appearance of gaps on the phonon transmittance spectrum, which are strongly correlated to the properties of the vibrational spectrum of the specific molecular species in the junction. The filtering effect results from a delicate interplay between the intrinsic vibrational structure of the molecular chains and the different Debye cutoffs of the nanoscopic electrodes used as thermal baths.
References
[1] Selective Transmission of Phonons in Molecular Junctions with Nanoscopic Thermal Baths Leonardo Medrano Sandonas, Álvaro Rodríguez Méndez, Rafael Gutierrez, Jesus M. Ugalde, Vladimiro Mujica, and Gianaurelio Cuniberti, J. Phys. Chem. C 2019, 123, 15, 9680-9687
Marco Garcia-Revilla
Departamento de Quimica, DCNE, Universidad de Guanajuato. 36050. Guanajuato. Mexico
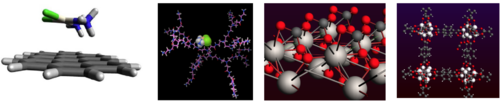
Chemical interactions in molecular systems: implications for drug delivery and environmental remediation
Binding belongs to the most important concepts in life sciences, material sciences and Chemistry. Such a concept is used to the rationalization of many phenomena as: chemical reactivity, structural stability, biological and pharmaceutical activity, optical properties and physicochemical properties of condensed mater. Nevertheless, binding is one of the most unknown concepts from the formal physical perspective and it is subject to ambiguities. The reason of this is that traditional chemical concepts, as is the case of binding, were defined and used decades before of the development of Quantum Mechanics. For this reason, the construction of approaches linking results of quantum mechanical treatments of molecular systems with traditional concepts is a popular research topic for the computational and theoretical chemistry community. The implications of such theoretical developments to the study of molecular systems are important to gain physicochemical insight and to rationalize the studied phenomena from the physicochemical perspective. Some examples of this implications are discussed in this presentation, as 1) the use of graphene to support hazardous anticancer agents, 2) the design of dendrimers(PAMAM) to deliver hazardous anticancer agents and 3) The absorption of CO2 and H2 in Metal Organic Frameworks. In this work, we analyze the non-covalent interactions of the systems cisplatin-graphene and cisplatin-PAMAM. We have use finite prototypes for graphene and PAMAM, for the case of PAMAM prototypes we have use molecular dynamic methodology to get the plausible conformations. In addition, the DFT Symmetry-Adapted Perturbation Theory,[1] the Quantum Theory of Atoms in Molecules,[2] and Energy Decomposition Analysis[3] approaches were used to study chemical interactions. The most important conclusions are that interactions are strong enough to use graphene as anti-cancer drug support [4,5] and that the system CisPt-PAMAM display interactions and dynamic properties that permits the transport and delivery of Cis-Pt in acid environments, as is displayed in cancer cells.[5] Finally, we have investigated the adsorption of CO2 in TiO2 surfaces (Rutile and Anatase) to get insight about the photocatalytic CO2 reduction. Finally, the perspective is to use such insight to investigate the substituent effect of the and CO2 photocatalytic properties of MOFs.
References [1] Jansen, G. Symmetry‐adapted perturbation theory based on density functional theory for noncovalent interactions. WIREs Comput Mol Sci, 4:(2014) 127-144. [2] R. F. W. Bader Atoms in Molecules. A Quantum Theory. Oxford University Press, New York, 1990. [3] F.M. Bickelhaupt et.al. Rev. Comput. Chem.; Lipkowitz, K. B. and Boyd, D. B., Eds.; Wiley-VCH: New York, 2000, Vol. 15, 1-86. [4] Cuevas‐Flores, M. D. R., Garcia‐Revilla, M. A., Bartolomei, M. J. Comput. Chem.2018, 39, 71– 80. [5] Cuevas‐Flores, M. D. R.(2018) “Computational Modeling and characterization of finite prototypes of nano-materials and its interaction with anticancer drugs” Doctoral Dissertation.
Joel Yuen-Zhou
University of California San Diego
Molecules in cavity: Polariton chemistry
Abstract Organic molecules interact strongly with confined electromagnetic fields in plasmonic arrays or optical microcavities owing to their bright transition dipole moments. This interaction gives rise to molecular polaritons, hybrid light-matter quasiparticles. Molecular polaritonics opens doors for new room-temperature opportunities for the nontrivial control of physico-chemical properties of molecular assemblies [1]. In this talk, I’ll showcase some of these opportunities that we have been theoretically (and, together with our experimental collaborators) exploring in the past few years. I will briefly discuss the relevant time and energy scales associated with molecular polaritons [1,2] and strategies to exploit them to control photoexcited processes including singlet fission [3], triplet harvesting, remote and topologically-protected energy transfer [4-6], and anomalous nonlinear optical effects [7,8]. Finally, I will conclude by explaining how vibrational polaritons can steer ground-state chemical reactions even in the absence of optical pumping [9], or be used to realize exotic processes such as remote control of chemical reactions [10].
References:
[1] R. F. Ribeiro, L. Martínez-Martínez, M. Du, and J. Yuen-Zhou, Polariton chemistry: controlling molecular dynamics with optical cavities, Chem. Sci. 9, 6325-6339 (2018). [2] L. A. Martínez-Martínez, R. F. Ribeiro, J. A. Campos-González-Angulo, and J. Yuen-Zhou, Can ultrastrong coupling change ground-state chemical reactions?, ACS Photonics 5, 167 (2018). [3] L. A. Martínez-Martínez, M. Du, R. F. Ribeiro, S. Kena-Cohen, and J. Yuen-Zhou, Polariton-assisted singlet fission in acene aggregates, J. Phys. Chem. Lett., 9, 1951-1957 (2018) (ACS editor’s choice). [4] M. Du, L. A. Martínez-Martínez, R. F. Ribeiro, Z. Hu, V. M. Menon, and J. Yuen-Zhou, Theory for polariton assisted remote energy transfer, Chem. Sci. 9, 6659-6669 (2018). [5] J. Yuen-Zhou, S. K. Saikin, T. Zhu, M. Onbalsi, C. Ross, V. Bulovic, and M. Baldo, Plexcitons: Dirac points and topological modes, Nat. Commun. 7, 11783 (2016). [6] J. Yuen-Zhou, S. Saikin, N. Yao, and A. Aspuru-Guzik, Topologically protected excitons in porphyrin thin films, Nature Mater. 13, 1026 (2014). [7] B. Xiang, R. F. Ribeiro, A. D. Dunkelberger, J. Wang, Y. Li, B. S. Simpkins, J. C. Owrutsky, J. Yuen-Zhou, W. Xiong, Two-dimensional spectroscopy of vibrational polaritons, Proc. Nat. Acad. Sci. 201722063 (2018). [8] R. F. Ribeiro, A. D. Dunkelberger, B. Xiang, W. Xiong, B. S. Simpkins, J. C. Owrutsky, J. Yuen-Zhou, Theory for nonlinear spectroscopy of vibrational polaritons, J. Phys. Chem. Lett. 9, 13, 3766--3771 (2018). [9] J. Campos-González-Angulo, R. F. Ribeiro, and J. Yuen-Zhou, Resonant enhancement of thermally-activated reactions via vibrational polaritons, arXiV:1902.10264. [10] M. Du, R. F. Ribeiro, J. Yuen-Zhou, Remote control of chemical reactions with optical cavities, Chem, 2019.
Eduardo Ludeña
Universidad San Francisco de Quito, Quito, Ecuador
How feasible is it to construct N-representable energy functionals in DFT?
We review the problem of functional N-representability in DFT and examine the close connection between Levy’s constrained search and the N-representability problem in 2-RDM theory [1] and make some comments on the status of this problem in DFT. [2] We discuss recent advances which incorporate N-representability conditions in 1-RDM theory as expounded, for example, in the Piris version of NOFT. [3] We also consider the recent inclusion of N-representability conditions in variational formulations of 2-RDM theory for particular cases of CI expansions as presented in the work of Alcoba et al. [4] In this context, we discuss the possibility of using local-scaling transformations [1] for generating N-representable DFT functionals derived from the 1-matrix and 2-matrix formulations.
References
[1] Kryachko, E. S., Ludeña, E. V. (2014). Density functional theory: Foundations reviewed. Physics Reports, 544(2), 123-239.
[2] P.W. Ayers, S. Liu, Necessary and sufficient conditions for the N-representability of density functionals, Phys. Rev. A 75 (2007) 022514.
[3] Mario Piris, in: Reduced-Density-Matrix Mechanics: With Application to Many-Electron Atoms and Molecules, Advances in Chemical Physics, Volume 134, 387, (2007), David A. Mazziotti (ed.); Piris, M., Ugalde, J. M. (2014). Perspective on natural orbital functional theory. International Journal of Quantum Chemistry, 114(18), 1169-1175.
[4] Alcoba, D. R., Torre, A., Lain, L., Massaccesi, G. E., Oña, O. B., & Ríos, E. (2019). Unrestricted treatment for the direct variational determination of the two-electron reduced density matrix for doubly occupied-configuration-interaction wave functions. J. Chem. Phys., 150(16), 164106.
Chao-Ping (Cherri) Hsu
Institute of Chemistry, Academia Sinica
Excitation Energies from Time-Dependent Thermally-Assisted-Occupation Density Functional Theory
The linear response time-dependent density functional theory (LR-TDDFT) has been broadly used to investigate excited-state properties of various molecular systems. However, current LR-TDDFT methods heavily rely upon outcomes from ground-state DFT calculations which may be prone to errors due to lack of treatment in the non-dynamical correlation effects with commonly used functionals. Recently, the thermally-assisted-occupation (TAO) DFT scheme [1,2] was proposed, which explicitly incorporates the non-dynamical correlation effect in the ground-state simulation, but retains the low computational complexity of conventional DFT. In this work, we develop the linear-response theory for TAO-DFT (TDTAO-DFT) to study excited states of H2. The correct feature of the first triplet excited state including the non-imaginary excitation energies, as well as zero singlet-triplet gap in the dissociation limit, are correctly reproduced by TDTAO-DFT. In addition, the overall excited-state potential energy surfaces (PESs) obtained from TDTAO-DFT also have excellent agreement with results from the state-of-the-art equation-of-motion coupled cluster singles doubles (EOM-CCSD) calculations.
References
[1] J.-D. Chai, J. Chem. Phys. 136, 154104 (2012).
[2] J.-D. Chai, J. Chem. Phys. 140, 18A521 (2014).
Weitao Yang
Department of Chemistry and Department of Physics, Duke University, USA
Eliminating the Delocalization and Static/Strong Correlation Error in Density Functional Approximations
The perspectives of fractional charges and fractional spins provide a clear analysis of the errors of commonly used density functional approximations (DFAs). These errors, the delocalization and static correlation error, lead to diversified problems in present-day density functional theory calculations. For achieving a universal elimination of these two errors, we develop a localized orbital scaling correction (LOSC) framework: it accurately characterizes the distributions of global and local fractional electrons and spins, and is thus capable of correcting system energy, energy derivative and electron density in a self-consistent and size-consistent manner. Our approach introduces the explicit derivative discontinuity and largely restores the flat-plane behavior of electronic energy at fractional charges and fractional spins. The LOSC–DFAs lead to systematically improved results, including the dissociation of ionic species, single bonds, multiple bonds without breaking the space or spin symmetry, the band gaps of molecules and polymer chains, the energy and density changes upon electron addition and removal, and photoemission spectra. We further carried the comparison of experimental quasiparticle energies for many finite systems with calculations from the GW Green function and the localized orbitals scaling correction (LOSC). Extensive results with over 40 systems clearly show that LOSC orbital energies achieve slightly better accuracy than the GW calculations with little dependence on the semilocal DFA, supporting the use of LOSC DFA orbital energies to predict quasiparticle energies. This also leads to the QE-DFT (quasiparticle energies from DFT) approach: the calculations of excitation energies of the N-electron systems from the ground state DFA calculations of the (N - 1)-electron systems. Results show good performance with accuracy similar to TDDFT and the delta SCF approach for valence excitations with commonly used DFAs with or without LOSC. For charge transfer and Rydberg states, good accuracy was obtained only with the use of LOSC DFA. The QE-DFT method has been further developed to describe excited-state potential energy surfaces (PESs), conical intersections, and the analytical gradients of excited-state PESs.
References
1. J. Cohen, P. Mori-Sanchez, and W. Yang. Insights into current limitations of density functional theory. Science, 321:792, 2008.
2. P. Mori-Sánchez, A. J. Cohen, and W. Yang, “Localization and Delocalization Errors in Density Functional Theory and Implications for Band-Gap Prediction,” Physical Review Letters, 100: 146401, 2008.
3. P. Mori-Sanchez, A. J. Cohen, and W. Yang. Discontinuous Nature of the Exchange-Correlation Functional in Strongly Correlated Systems, Physical Review Letters, 102:066403, 2009.
4. J. Cohen, P. Mori-Sanchez, and W. Yang. Challenges for Density Functional Theory. Chem. Rev. 112:289, 2012
5. C. Li, X. Zheng, N. Q. Su, and W. Yang, “Localized orbital scaling correction for systematic elimination of delocalization error in density functional approximations,” National Science Review, 5: 203–215, 2018.
6. N. Q. Su, C. Li, and W. Yang, “Describing strong correlation with fractional-spin correction in density functional theory,” Proceedings of the National Academy of Sciences, 115:9678–9683, 2018.
7. Y. Mei, C. Li, N. Q. Su, and W. Yang, “Approximating Quasiparticle and Excitation Energies from Ground State Generalized Kohn-Sham Calculations,” arXiv:1810.09906 2018; J. Phys. Chem. A, 123(3), 666–673, 2019
8. Y. Mei and W. Yang, “Charge transfer excitation energies from ground state density functional theory calculations,” J. Chem. Phys., 150, 144109, 2019.
9. Y. Mei and W. Yang, “Excited-State Potential Energy Surfaces, Conical Intersections, and Analytical Gradients from Ground-State Density Functional Theory,” J. Phys. Chem. Lett. 10, 2538–2545, 2019.
Farnaz Heidar-Zadeh
Center for Molecular Modeling (CMM), Ghent University, Ghent, Belgium.
Unravelling Atoms in Molecules: A Theoretical Perspective
It is common to use the electron density to partition a system into atomic regions. The necessity for such a partitioning scheme is rooted in the unquestionable role of atoms in chemistry. Nevertheless, atomic properties are not well-defined concepts within the domain of quantum mechanics, as they are not physical observables. To resolve this ambiguity, we use information theory to find the atoms-in-a-molecule that most strongly resemble reference atoms.[1-4]
First, we justify the popular Hirshfeld partitioning. Specifically, we prove that the family of f-divergence measures as necessary and sufficient for deriving Hirshfeld partitioning.[3] To choose between the various possible Hirshfeld methods, we impose a physical constraint, namely that the when one partitions two well-separated non-interacting sub-systems, one obtains the same result that one would obtain when the subsystems are treated separately. Then, among all possible f-divergences, only the (extended) Kullback-Leibler information measure can be used. This gives a new partitioning method, which we call the Additive Variational Hirshfeld (AVH) partitioning. In AVH, one defines a “promolecular” density, which is expanded as a convex linear com-bination of spherically-averaged charged- and uncharged-densities for isolated atoms; the expansion coefficients are determined by minimizing the extended Kullback-Leibler divergence between the true molecular density and its promolecular approximation. The robustness of AVH is confirmed by testing it on various datasets. Considering its unique mathematical properties and our favorable numerical results, we believe that AVH has the potential to supplant other partitioning methods.
References
[1] F. Heidar-Zadeh F., P.W. Ayers, T. Verstraelen, I. Vinogradov, E. Vöhringer-Martinez, and P. Bultinck, "Information-theoretic approaches to atoms-in-molecules: Hirshfeld family of partitioning schemes", J. Phys. Chem. A. pp. 4219-4245 (2017).
[2] F. Heidar-Zadeh, P.W. Ayers, and P. Bultinck, "Deriving the Hirshfeld partitioning using distance metrics", J. Chem. Phys. 141, 094103 (2014).
[3] F. Heidar-Zadeh, and P.W. Ayers, "How pervasive is the Hirshfeld partitioning?", J. Chem. Phys. 142, 044107 (2015).
[4] T. Verstraelen, S. Vandenbrande, F. Heidar-Zadeh, L. Vanduyfhuys, V. Van Speybroek, M. Waroquier, and P.W. Ayers, "Minimal basis iterative stockholder: atoms in molecules for force-field development", J. Chem. Theory Comput. 12, pp. 3894-3912 (2016).
Balazs Pinter
Department of Chemistry, Universidad Técnica Federico Santa María (USM), Valparaíso, Chile
DFT investigation of the oxidative and reductive quenching cycles of ruthenium photoredox catalysts
In the last few years, efficient and controlled photoredox catalysis has become a main target for academia and industry due to its potential applications in driving the transformations of feedstock chemicals using sunlight as an energy source [1]. Photoredox catalysts based on ruthenium and iridium are still amongst the most promising candidates to achieve such goals on the short run. The absorption of light by these systems generates a low-energy hole on the metal and a high-energy electron on the ligand, as depicted below, making the excited-state molecules participate in high-energy electron transfer reactions, facilitated also by the long-lifetime of the excited triplet MLCT state.
While computing redox potentials and modeling excited states of TM complexes remain challenging in general, our computational protocol based on Density Functional Theory coupled to re-parameterized implicit solvation (SMD) provide accurate redox potentials (typically within 0.1 V) for both excited- and ground state oxidations and reductions of these complexes. In contrast to earlier studies that utilizes TD-DFT to compute the relative stability of the excited MLCT state [2], we directly access the energy of the triplet MLCT state using standard ground state DFT making the computational protocol robust and predictive paving the way to rational design of these photoredox catalysts through large-scale computational studies. This computational method will be presented in detail, discussed for 4 different redox transitions of 25 ruthenium complexes and contrasted with available experimental data. Ligand design strategies and mechanisms to localize the radical on one ligand as well as to enhance the lifespan of the reactive triplet-state will be also discussed.
References
[1] J. A. Terrett, J. D. Cuthbertson, V. W.Shurtleff and D. W. C. MacMillan, Nature, 524, 330, (2015).
[2] T. B. Demissie, K. Ruud and J. H. Hansen, Organometallics, 34, 4218 (2015).
Shubin Liu
University of North Carolina at Chapel Hill
Quantification and origin of cooperativity: Insights from density functional theory and information-theoretic approach
Cooperativity is a widely used chemical concept whose existence is ubiquitous in chemical and biological systems but whose quantification is still controversial and origin much less appreciated. In this work, using the interaction energy of a molecular system, which is composed of multiple copies of a building block, we propose a quantitative measurement to evaluate the cooperativity effect. This quantification approach is then applied to a few molecular systems, i.e., water cluster, argon cluster, protonated water cluster, zinc atom cluster, water cluster on top of a graphene sheet, and alpha helix of glycine amino acids, each with up to 20 copies of the building block. Cooperativity is seen in all these systems. Both positive and negative cooperativity effects are observed. Employing the two energy partition schemes in density functional theory and the information-theoretic quantities such as Shannon entropy, Fisher information, information gain, etc., we then examine the origin of the cooperativity effect for these systems. Strong linear correlations between the cooperativity measure and some of these theoretical quantities have been unveiled. With these correlations, we are able to quantitatively account for their origin of cooperativity. Our results show that the interactions governing the existence and validity of the cooperativity effect are complicated. An opposite mechanism in enthalpy–entropy compensation for positive and negative cooperativity has been unveiled. These results should provide new insights and understandings from a different viewpoint about the nature and origin of cooperativity to appreciate this vastly important chemical concept.
References
1. Chunying Rong, Dongbo Zhao, Donghai Yu, and Shubin Liu, Phys. Chem. Chem. Phys. 20, 17990-17998 (2018).
2. Tiangjing Zhou, Siyuan Liu, Donghai Yu, Dongbo Zhao, Chunying Rong, and Shubin Liu, Chem. Phys. Lett. 716, 192 – 198 (2019).
3. Chunying Rong, Dongbo Zhao, Tianjing Zhou, Siyuan Liu, Donghai Yu, and Shubin Liu, J. Phys. Chem. Lett., 10, 1716–1721 (2019).